Dissolved Trace Metals and Organic Matter Distribution in the Northern Adriatic, an Increasingly Oligotrophic Shallow Sea
Abstract
:1. Introduction
2. Materials and Methods
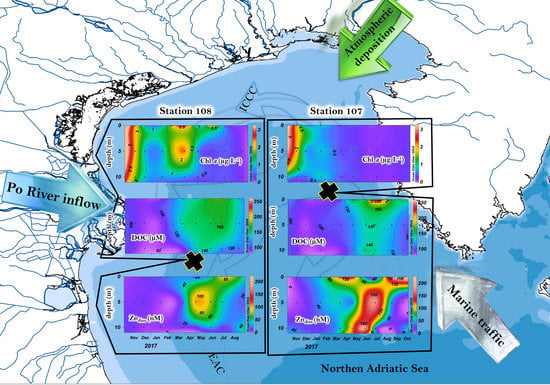
2.1. Study Area and Sampling
2.2. Physico-Chemical Parameters
2.3. Nutrient and Chlorophyll a Analysis
2.4. Organic Matter Characterization
2.5. Trace Metal Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Parameters of the Northern Adriatic
3.2. Nutrient Regime in the Northern Adriatic
3.3. Trace Metal Distribution in the Northern Adriatic
3.4. Dissolved Organic Matter Distribution in the NA
3.5. DOM Impacts on the Distribution of TMs in the Northern Adriatic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunda, W.G. Feedback interactions between trace metal nutrients and phytoplankton in the ocean. Front. Microbiol. 2012, 3, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richir, J. Trace Elements in Marine Environments: Occurrence, Threats and Monitoring with Special Focus on the Coastal Mediterranean. J. Environ. Anal. Toxicol. 2016, 6, 1000349. [Google Scholar] [CrossRef] [Green Version]
- Wolter, K.; Rabsch, U.; Krischker, P.; Davies, A. Influence of low concentrations of cadmium, copper and zinc on phytoplankton of natural water samples. Mar. Ecol. Prog. Ser. 1984, 19, 167–173. [Google Scholar] [CrossRef]
- Lee, J.G.; Morel, F.M.M. Replacement of zinc by cadmium in marine phytoplankton. Mar. Ecol. Prog. Ser. 1995, 127, 305–309. [Google Scholar] [CrossRef]
- Mason, R.P. Trace Metals in Aquatic Systems; Wiley-Blackwell: West Sussex, UK, 2013; ISBN 9781405160483. [Google Scholar]
- Morel, F.M.M.; Miligan, A.J.; Saito, M.A. Marine Bioinorganic Chemistry: The Role of Trace Metals in the Oceanic Cycles of Major Nutrients. In The Oceans and Marine Geochemistry; Elderfield, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 6, pp. 113–143. [Google Scholar]
- Ure, A.M.; Davidson, C.M. Introduction. In Chemical Speciation in the Environment, 2nd ed.; Wiley-Blackwell: West Sussex, UK, 2007. [Google Scholar]
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal Metallothioneins and Phytochelatins and Their Potential Use in Bioremediation. Front. Microbiol. 2020, 11, 517. [Google Scholar] [CrossRef]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L.; et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Semeniuk, D.M.; Bundy, R.M.; Payne, C.D.; Barbeau, K.A.; Maldonado, M.T. Acquisition of organically complexed copper by marine phytoplankton and bacteria in the northeast subarctic Pacific Ocean. Mar. Chem. 2015, 173, 222–233. [Google Scholar] [CrossRef]
- Zoppini, A.; Pettine, M.; Totti, C.; Puddu, A.; Artegiani, A.; Pagnotta, R. Nutrients, standing crop and primary production in western coastal waters of the adriatic sea. Estuar. Coast. Shelf Sci. 1995, 41, 493–513. [Google Scholar] [CrossRef]
- Vezzoli, R.; Mercogliano, P.; Pecora, S.; Zollo, A.L.; Cacciamani, C. Hydrological simulation of po river (North Italy) discharge under climate change scenarios using the RCM COSMO-CLM. Sci. Total Environ. 2015, 521–522, 346–358. [Google Scholar] [CrossRef]
- Rossini, P.; Guerzoni, S.; Molinaroli, E.; Rampazzo, G.; De Lazzari, A.; Zancanaro, A. Atmospheric bulk deposition to the lagoon of Venice: Part, I. Fluxes of metals, nutrients and organic contaminants. Environ. Int. 2005, 31, 959–974. [Google Scholar] [CrossRef] [Green Version]
- Djakovac, T.; Degobbis, D.; Supić, N.; Precali, R. Marked reduction of eutrophication pressure in the northeastern Adriatic in the period 2000–2009. Estuar. Coast. Shelf Sci. 2012, 115, 25–32. [Google Scholar] [CrossRef]
- Novak, T.; Godrijan, J.; Pfannkuchen, D.M.; Djakovac, T.; Medić, N.; Ivančić, I.; Mlakar, M.; Gašparović, B. Global warming and oligotrophication lead to increased lipid production in marine phytoplankton. Sci. Total Environ. 2019, 668, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Grilli, F.; Accoroni, S.; Acri, F.; Aubry, F.B.; Bergami, C.; Cabrini, M.; Campanelli, A.; Giani, M.; Guicciardi, S.; Marini, M.; et al. Seasonal and interannual trends of oceanographic parameters over 40 years in the northern Adriatic Sea in relation to nutrient loadings using the EMODnet chemistry data portal. Water 2020, 12, 2280. [Google Scholar] [CrossRef]
- Gašparović, B.; Djakovac, T.; Tepić, N.; Degobbis, D. Relationships between surface-active organic substances, chlorophyll a and nutrients in the northern Adriatic Sea. Cont. Shelf Res. 2011, 31, 1149–1160. [Google Scholar] [CrossRef]
- Dautović, J.; Vojvodić, V.; Tepić, N.; Ćosović, B.; Ciglenečki, I. Dissolved organic carbon as potential indicator of global change: A long-term investigation in the northern Adriatic. Sci. Total Environ. 2017, 587–588, 185–195. [Google Scholar] [CrossRef]
- Reisenhofer, E.; Adami, G.; Favretto, A. Heavy metals and nutrients in coastal surface seawaters (Gulf of Trieste, Northern Adriatic Sea): An environmental study by factor analysis. Fresenius J. Anal. Chem. 1996, 354, 729–734. [Google Scholar] [CrossRef]
- Martinčič, D.; Kwokal, Ž.; Stoeppler, M.; Branica, M. Trace metals in sediments from the adriatic sea. Sci. Total Environ. 1989, 84, 135–147. [Google Scholar] [CrossRef]
- Martinčić, D.; Kwokal, Ž.; Branica, M. Distribution of zinc, lead, cadmium and copper between different size fractions of sediments I. The Limski Kanal (North Adriatic Sea). Sci. Total Environ. 1990, 95, 201–215. [Google Scholar] [CrossRef]
- Sfriso, A.A.; Chiesa, S.; Sfriso, A.; Buosi, A.; Gobbo, L.; Boscolo Gnolo, A.; Argese, E. Spatial distribution, bioaccumulation profiles and risk for consumption of edible bivalves: A comparison among razor clam, Manila clam and cockles in the Venice Lagoon. Sci. Total Environ. 2018, 643, 579–591. [Google Scholar] [CrossRef]
- Zago, C.; Capodaglio, G.; Barbante, C.; Giani, M.; Moret, I.; Scarponi, G.; Cescon, P. Heavy metal distribution and speciation in the northern Adriatic Sea. Chem. Ecol. 2002, 18, 39–51. [Google Scholar] [CrossRef]
- Zago, C.; Capodaglio, G.; Ceradini, S.; Ciceri, G.; Abelmoschi, L.; Soggia, F.; Cescon, P.; Scarponi, G. Benthic fluxes of cadmium, lead, copper and nitrogen species in the northern Adriatic Sea in front of the River Po outflow, Italy. Sci. Total Environ. 2000, 246, 121–137. [Google Scholar] [CrossRef]
- Pavoni, E.; Crosera, M.; Petranich, E.; Adami, G.; Faganeli, J.; Covelli, S. Partitioning and mixing behaviour of trace elements at the Isonzo/Soča River mouth (Gulf of Trieste, northern Adriatic Sea). Mar. Chem. 2020, 223, 103800. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Truzzi, C.; Tercier-Waeber, M.L.; Nöel, S.; Braungardt, C.B.; Achterberg, E.P.; Howell, K.A.; Turner, D.; Marini, M.; et al. In-situ trace metal (Cd, Pb, Cu) speciation along the Po River plume (northern Adriatic Sea) using submersible systems. Mar. Chem. 2019, 212, 47–63. [Google Scholar] [CrossRef]
- Tankéré, S.P.C.; Price, N.B.; Statham, P.J. Mass balance of trace metals in the Adriatic Sea. J. Mar. Syst. 2000, 25, 269–286. [Google Scholar] [CrossRef]
- Gašparović, B.; Godrijan, J.; Frka, S.; Tomažić, I.; Penezić, A.; Marić, D.; Djakovac, T.; Ivančić, I.; Paliaga, P.; Lyons, D.; et al. Adaptation of marine plankton to environmental stress by glycolipid accumulation. Mar. Environ. Res. 2013, 92, 120–132. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Toronto, ON, Canada, 1984. [Google Scholar]
- Ivančić, I.; Degobbis, D. Mechanisms of production and fate of organic phosphorus in the northern Adriatic Sea. Mar. Biol. 1987, 94, 117–125. [Google Scholar] [CrossRef]
- Ivančić, I.; Degobbis, D. An optimal manual procedure for ammonia analysis in natural waters by the indophenol blue method. Water Res. 1984, 18, 1143–1147. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 2nd ed.; John Wiley & Sons: New York, USA, 2000; ISBN 0471282723. [Google Scholar]
- Plavšić, M.; Strmečki, S. Carbohydrate polymers as constituents of exopolymer substances in seawater, their complexing properties towards copper ions, surface and catalytic activity determined by electrochemical methods. Carbohydr. Polym. 2016, 135, 48–56. [Google Scholar] [CrossRef]
- Strmečki, S.; Dautović, J.; Plavšić, M. Constant current chronopotentiometric stripping characterisation of organic matter in seawater from the northern Adriatic, Croatia. Environ. Chem. 2014, 11, 158–166. [Google Scholar] [CrossRef]
- Strmečki, S.; Pereža, L. Electrochemistry of chitosan amino-glycan and BSA protein mixture under seawater conditions. J. Electroanal. Chem. 2021, 898, 115630. [Google Scholar] [CrossRef]
- Cuculić, V.; Cukrov, N.; Kwokal, Ž.; Mlakar, M. Natural and anthropogenic sources of Hg, Cd, Pb, Cu and Zn in seawater and sediment of Mljet National Park, Croatia. Estuar. Coast. Shelf Sci. 2009, 81, 311–320. [Google Scholar] [CrossRef]
- Cindrić, A.M.; Garnier, C.; Oursel, B.; Pižeta, I.; Omanović, D. Evidencing the natural and anthropogenic processes controlling trace metals dynamic in a highly stratified estuary: The Krka River estuary (Adriatic, Croatia). Mar. Pollut. Bull. 2015, 94, 199–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cozzi, S.; Ibáñez, C.; Lazar, L.; Raimbault, P.; Giani, M. Flow regime and nutrient-loading trends from the largest South European watersheds: Implications for the productivity of mediterranean and Black Sea’s Coastal Areas. Water 2018, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Cardin, V.; Ursella, L. Hydrological Characteristics and Dynamics of the Northern Adriatic during Late Summer and Autumn 1997. In Mediterranean Ecosystems: Structures and Processes; Faranda, F.M., Guglielmo, L., Spezie, G., Eds.; Springer: Milan, Italy, 2001; pp. 25–35. [Google Scholar]
- Franco, P. Osservazioni sull’oceanografia fisica e chimica dell’Adriatico. In Atti del Convegno-Lo stato di salute dell’Adriatico. Problemi e Prospettive; Sogesta: Urbino, Italy, 1989; pp. 21–29. [Google Scholar]
- Degobbis, D.; Gilmartin, M. Nitrogen, phosphorus, and biogenic silicon budgets for the northern Adriatic Sea. Oceanol. Acta 1990, 13, 31–45. [Google Scholar]
- Poulain, P.-M.; Kourafalou, V.H.; Cushman-Roisin, B. Northern Adriatic Sea. In Physical Oceanography of the Adriatic Sea: Past, Present and Future; Cushman-Roisin, B., Gačić, M., Poulain, P.-M., Artegiani, A., Eds.; Springer Science+Business Media: Dordrecht, The Netherlands, 2001; pp. 143–165. [Google Scholar]
- Supić, N.; Orlić, M.; Degobbis, D. Istrian Coastal Countercurrent and its year-to-year variability. Estuar. Coast. Shelf Sci. 2000, 51, 385–397. [Google Scholar] [CrossRef]
- Supić, N.; Degobbis, D.; Dakovac, T.; Krajcar, V.; Precali, R.; Orlić, M. Occurrence of the Istrian Coastal Countercurrent in 2000, a year with a mucilage event. Geofizika 2001, 18–19, 45–57. [Google Scholar]
- Marić, D.; Kraus, R.; Godrijan, J.; Supić, N.; Djakovac, T.; Precali, R. Phytoplankton response to climatic and anthropogenic influences in the north-eastern Adriatic during the last four decades. Estuar. Coast. Shelf Sci. 2012, 115, 98–112. [Google Scholar] [CrossRef]
- Gašparović, B. Decreased production of surface-active organic substances as a consequence of the oligotrophication in the northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 33–39. [Google Scholar] [CrossRef]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent changes in the marine ecosystems of the northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Novak, T.; Godrijan, J.; Pfannkuchen, D.M.; Djakovac, T.; Mlakar, M.; Baricevic, A.; Tanković, M.S.; Gašparović, B. Enhanced dissolved lipid production as a response to the sea surface warming. J. Mar. Syst. 2018, 180, 289–298. [Google Scholar] [CrossRef]
- Cozzi, S.; Lipizer, M.; Cantoni, C.; Catalano, G. Nutrient balance in the ecosystem of the North Western Adriatic Sea. Chem. Ecol. 2002, 18, 1–12. [Google Scholar] [CrossRef]
- Degobbis, D.; Precali, R.; Ivancic, I.; Smodlaka, N.; Fuks, D.; Kveder, S. Long-term changes in the northern Adriatic ecosystem related to anthropogenic eutrophication. Int. J. Environ. Pollut. 2000, 13, 495–533. [Google Scholar] [CrossRef]
- Cozzi, S.; Giani, M. River water and nutrient discharges in the northern Adriatic Sea: Current importance and long term changes. Cont. Shelf Res. 2011, 31, 1881–1893. [Google Scholar] [CrossRef]
- Berman, T.; Béchemin, C.; Maestrini, S.Y. Release of ammonium and urea from dissolved organic nitrogen in aquatic ecosystems. Aquat. Microb. Ecol. 1999, 16, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Ivančić, I.; Pfannkuchen, M.; Godrijan, J.; Djakovac, T.; Marić Pfannkuchen, D.; Korlević, M.; Gašparović, B.; Najdek, M. Alkaline phosphatase activity related to phosphorus stress of microphytoplankton in different trophic conditions. Prog. Oceanogr. 2016, 146, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Celussi, M.; Del Negro, P. Microbial degradation at a shallow coastal site: Long-term spectra and rates of exoenzymatic activities in the NE Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 75–86. [Google Scholar] [CrossRef]
- Pettine, M.; Camusso, M.; Martinotti, W.; Marchetti, R.; Passino, R.; Queirazza, G. Soluble and particulate metals in the Po River: Factors affecting concentrations and partitioning. Sci. Total Environ. 1994, 145, 243–265. [Google Scholar] [CrossRef]
- Tankere, S.P.C.; Statham, P.J. Distribution of dissolved Cd, Cu, Ni and Zn in the Adriatic Sea. Mar. Pollut. Bull. 1996, 32, 623–630. [Google Scholar] [CrossRef]
- Cuculić, V.; Cukrov, N.; Kwokal, Ž.; Strmečki, S.; Plavšić, M. Assessing trace metal contamination and organic matter in the brackish lakes as the major source of potable water. Environ. Geochem. Health 2018, 40, 489–503. [Google Scholar] [CrossRef]
- Oursel, B.; Garnier, C.; Durrieu, G.; Mounier, S.; Omanović, D.; Lucas, Y. Dynamics and fates of trace metals chronically input in a Mediterranean coastal zone impacted by a large urban area. Mar. Pollut. Bull. 2013, 69, 137–149. [Google Scholar] [CrossRef]
- Yoon, Y.Y.; Martin, J.M.; Cotté, M.H. Dissolved trace metals in the Western Mediterranean Sea: Total concentration and fraction isolated by C18 Sep-Pak technique. Mar. Chem. 1999, 66, 129–148. [Google Scholar] [CrossRef]
- Chifflet, S.; Tedetti, M.; Zouch, H.; Fourati, R.; Zaghden, H.; Elleuch, B.; Quéméneur, M.; Karray, F.; Sayadi, S. Dynamics of trace metals in a shallow coastal ecosystem: Insights from the Gulf of Gabès (southern Mediterranean Sea). AIMS Environ. Sci. 2019, 6, 277–297. [Google Scholar] [CrossRef]
- Plavšić, M.; Ćosović, B. Influence of surface-active substances on the redox processes of metal ions: A contribution to the speciation analysis of metals in aquatic systems. Anal. Chim. Acta 1994, 284, 539–545. [Google Scholar] [CrossRef]
- Carr, N.; Davis, C.E.; Blackbird, S.; Daniels, L.R.; Preece, C.; Woodward, M.; Mahaffey, C. Seasonal and spatial variability in the optical characteristics of DOM in a temperate shelf sea. Prog. Oceanogr. 2018, 177, 101929. [Google Scholar] [CrossRef]
- Jiao, N.; Robinson, C.; Azam, F.; Thomas, H.; Baltar, F.; Dang, H.; Hardman-Mountford, N.J.; Johnson, M.; Kirchman, D.L.; Koch, B.P.; et al. Mechanisms of microbial carbon sequestration in the ocean–future research directions. Biogeosciences 2014, 11, 5285–5306. [Google Scholar] [CrossRef] [Green Version]
- Tepić, N.; Gašparović, B.; Ahel, M. Multivariate statistical analysis of the distribution patterns of carbohydrates and surface-active substances in the northern Adriatic Sea. Mar. Chem. 2009, 114, 37–46. [Google Scholar] [CrossRef]
- Gašparović, B.; Plavšić, M.; Ćosović, B.; Saliot, A. Organic matter characterization in the sea surface microlayers in the subarctic Norwegian fjords region. Mar. Chem. 2007, 105, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gašparović, B.; Vojvodić, V.; Ćosović, B. Excretion of Organic Matter during an Experimental Phytoplankton Bloom Followed Using o-Nitrophenol as an Electrochemical Probe. Croat. Chem. Acta 1998, 71, 271–284. [Google Scholar]
- Sunda, W.G. Trace metal interactions with marine phytoplankton. Biol. Oceanogr. 1988, 6, 411–442. [Google Scholar]
- Bruland, K.W. Complexation of zinc by natural organic ligands in the central North Pacific. Limnol. Oceanogr. 1989, 34, 269–285. [Google Scholar] [CrossRef] [Green Version]
- Domingos, R.F.; Gélabert, A.; Carreira, S.; Cordeiro, A.; Sivry, Y.; Benedetti, M.F. Metals in the Aquatic Environment—Interactions and Implications for the Speciation and Bioavailability: A Critical Overview. Aquat. Geochem. 2015, 21, 231–257. [Google Scholar] [CrossRef]
- Williams, P.M. Organic Compounds in Aquatic Environments; Faust, S.D., Hunter, J.V., Eds.; Dekker: New York, NY, USA, 1971; Chapter 7. [Google Scholar]
- Wada, S.; Suzuki, S. Inhibitory effect of zinc on the remineralisation of dissolved organic matter in the coastal environment. Aquat. Microb. Ecol. 2011, 63, 47–59. [Google Scholar] [CrossRef]
- Zhang, T.; Fiamingo, M.; Allen, H.C. Trace Metal Enrichment Driven by Phosphate Functional Group Binding Selectivity. J. Geophys. Res. Ocean. 2018, 123, 5286–5297. [Google Scholar] [CrossRef]
- Ingri, J.; Widerlund, A.; Suteerasak, T.; Bauer, S.; Elming, S.Å. Changes in trace metal sedimentation during freshening of a coastal basin. Mar. Chem. 2014, 167, 2–12. [Google Scholar] [CrossRef]
- Soon, Z.Y.; Jung, J.H.; Jang, M.; Kang, J.H.; Jang, M.C.; Lee, J.S.; Kim, M. Zinc Pyrithione (ZnPT) as an Antifouling Biocide in the Marine Environment—A Literature Review of Its Toxicity, Environmental Fates, and Analytical Methods. Water. Air. Soil Pollut. 2019, 230, 310. [Google Scholar] [CrossRef]
- Ytreberg, E.; Karlsson, J.; Eklund, B. Comparison of toxicity and release rates of Cu and Zn from anti-fouling paints leached in natural and artificial brackish seawater. Sci. Total Environ. 2010, 408, 2459–2466. [Google Scholar] [CrossRef]
- Miller, R.J.; Adeleye, A.S.; Page, H.M.; Kui, L.; Lenihan, H.S.; Keller, A.A. Nano and traditional copper and zinc antifouling coatings: Metal release and impact on marine sessile invertebrate communities. J. Nanopart. Res. 2020, 22, 129. [Google Scholar] [CrossRef]
- Contini, D.; Belosi, F.; Gambaro, A.; Cesari, D.; Stortini, A.M.; Bove, M.C. Comparison of PM10 concentrations and metal content in three different sites of the Venice Lagoon: An analysis of possible aerosol sources. J. Environ. Sci. 2012, 24, 1954–1965. [Google Scholar] [CrossRef] [Green Version]
- Rossini, P.; Guerzoni, S.; Rampazzo, G.; Quarantatto, G.; Garibbo, E.; Molinaroli, E. Atmospheric Deposition of Trace Metals in North Adriatic Sea. In Mediterranean Ecosystems; Faranda, F.M., Guglielmo, L., Spezie, G., Eds.; Springer: Milan, Italy, 2001; pp. 123–129. [Google Scholar]
- Heimbürger, L.E.; Migon, C.; Cossa, D. Impact of atmospheric deposition of anthropogenic and natural trace metals on Northwestern Mediterranean surface waters: A box model assessment. Environ. Pollut. 2011, 159, 1629–1634. [Google Scholar] [CrossRef] [Green Version]
- Bruland, K.W. Complexation of cadmium by natural organic ligands in the central North Pacific. Limnol. Oceanogr. 1992, 37, 1008–1017. [Google Scholar] [CrossRef]
- Horner, T.J.; Lee, R.B.Y.; Henderson, G.M.; Rickaby, R.E.M. Nonspecific uptake and homeostasis drive the oceanic cadmium cycle. Proc. Natl. Acad. Sci. USA 2013, 110, 2500–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, F.M.M. The oceanic cadmium cycle: Biological mistake or utilization? Proc. Natl. Acad. Sci. USA 2013, 110, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlitzer, R.; Anderson, R.F.; Masferrer Dodas, E.; Lohan, M.; Geibert, W.; Tagliabue, A.; Bowie, A.; Jeandel, C.; Maldonado, M.T.; Landing, W.M.; et al. The GEOTRACES Intermediate Data Product 2017. Chem. Geol. 2018, 493, 210–223. [Google Scholar] [CrossRef]
- Middag, R.; van Heuven, S.M.A.C.; Bruland, K.W.; de Baar, H.J.W. The relationship between cadmium and phosphate in the Atlantic Ocean unravelled. Earth Planet. Sci. Lett. 2018, 492, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Vance, D.; Little, S.H.; Archer, C.; Cameron, V.; Andersen, M.B.; Rijkenberg, M.J.A.; Lyons, T.W. The oceanic budgets of nickel and zinc isotopes: The importance of sulfidic environments as illustrated by the Black Sea. Philos. Trans. Royal Soc. A 2016, 374, 20150294. [Google Scholar] [CrossRef]
- Hassler, C.S.; Ridgway, K.R.; Bowie, A.R.; Butler, E.C.V.; Clementson, L.A.; Doblin, M.A.; Davies, D.M.; Law, C.; Ralph, P.J.; van der Merwe, P.; et al. Primary productivity induced by iron and nitrogen in the Tasman Sea: An overview of the PINTS expedition. Mar. Freshw. Res. 2014, 65, 517–537. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.C.; Galer, S.J.G.; Abouchami, W.; Rijkenberg, M.J.A.; de Baar, H.J.W.; De Jong, J.; Andreae, M.O. Non-Rayleigh control of upper-ocean Cd isotope fractionation in the western South Atlantic. Earth Planet. Sci. Lett. 2017, 471, 94–103. [Google Scholar] [CrossRef]
- Farkas, A.; Erratico, C.; Viganò, L. Assessment of the environmental significance of heavy metal pollution in surficial sediments of the River Po. Chemosphere 2007, 68, 761–768. [Google Scholar] [CrossRef]
- Sánchez-Marín, P.; Lorenzo, J.I.; Blust, R.; Beiras, R. Humic acids increase dissolved lead bioavailability for marine invertebrates. Environ. Sci. Technol. 2007, 41, 5679–5684. [Google Scholar] [CrossRef]
- Loaëc, M.; Olier, R.; Guezennec, J. Chelating properties of bacterial exopolysaccharides from deep-sea hydrothermal vents. Carbohydr. Polym. 1998, 35, 65–70. [Google Scholar] [CrossRef]
- Croot, P.L.; Hunter, K.A. Trace metal distributions across the continental shelf near Otago Peninsula, New Zealand. Mar. Chem. 1998, 62, 185–201. [Google Scholar] [CrossRef]
- Whitby, H.; Posacka, A.M.; Maldonado, M.T.; van den Berg, C.M.G. Copper-binding ligands in the NE Pacific. Mar. Chem. 2018, 204, 36–48. [Google Scholar] [CrossRef]
- Moffett, J.W.; Brand, L.E. Production of strong, extracellular Cu chelators by marine cyanobacteria in response to Cu stress. Limnol. Oceanogr. 1996, 41, 388–395. [Google Scholar] [CrossRef]
- Sunda, W.G.; Hanson, P.J. Chemical speciation of copper in river water—Effects of total copper, pH, carbonate, and dissolved organic matter. In Chemical Modeling in Aqueous Systems; Jenne, E.A., Ed.; American Chemical Society: Washington, DC, USA, 1979; pp. 147–180. [Google Scholar]
- Kozarac, Z.; Plavŝić, M.; Ćosović, B.; Viliĉić, D. Interaction of cadmium and copper with surface-active organic matter and complexing ligands released by marine phytoplankton. Mar. Chem. 1989, 26, 313–330. [Google Scholar] [CrossRef]
- Plavšić, M.; Kwokal, Ž.; Strmečki, S.; Peharec, Ž.; Omanović, D.; Branica, M. Determination of the copper complexing ligands in the Krka River estuary. Fresenius Environ. Bull. 2009, 18, 327–334. [Google Scholar]
- Plavšić, M.; Gašparović, B.; Strmečki, S.; Vojvodić, V.; Tepić, N. Copper complexing ligands and organic matter characteriza-tion in the northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2009, 85, 299–306. [Google Scholar] [CrossRef]
- Strmečki, S.A.; Plavšić, M.; Steigenberger, S.; Passow, U. Characterization of phytoplankton exudates and carbohydrates in relation to their complexation of copper, cadmium and iron. Mar. Ecol. Prog. Ser. 2010, 408, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Gikas, P. Single and combined effects of nickel (Ni(II)) and cobalt (Co(II)) ions on activated sludge and on other aerobic micro-organisms: A review. J. Hazard. Mater. 2008, 159, 187–203. [Google Scholar] [CrossRef]
- Bown, J.; Boye, M.; Nelson, D.M. New insights on the role of organic speciation in the biogeochemical cycle of dissolved cobalt in the southeastern Atlantic and the Southern Ocean. Biogeosciences 2012, 9, 2719–2736. [Google Scholar] [CrossRef] [Green Version]
- Bačinić, A.; Tumir, L.M.; Mlakar, M. Electrochemical characterization of Cobalt(II)-Complexes involved in marine biogeochemical processes. I. Co(II)-4-nitrocatechol and Co(II)-Humate. Electrochim. Acta 2020, 337, 135797. [Google Scholar] [CrossRef]
- Fuks, D.; Ivančić, I.; Najdek, M.; Lučić, D.; Njire, J.; Godrijan, J.; Marić, D.; Šilović, T.; Paliaga, P.; Blažina, M.; et al. Changes in the planktonic community structure related to trophic conditions: The case study of the northern Adriatic Sea. J. Mar. Syst. 2012, 96–97, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Nimmo, M.; van den Berg, C.M.G.; Brown, J. The chemical speciation of dissolved nickel, copper, vanadium and iron in Liv-erpool Bay, Irish Sea. Estuar. Coast. Shelf Sci. 1989, 29, 57–74. [Google Scholar] [CrossRef]
- de Carvalho, L.M.; Hollister, A.P.; Trindade, C.; Gledhill, M.; Koschinsky, A. Distribution and size fractionation of nickel and cobalt species along the Amazon estuary and mixing plume. Mar. Chem. 2021, 236, 104019. [Google Scholar] [CrossRef]
- Rees, T.A.V.; Bekheet, I.A. The role of nickel in urea assimilation by algae. Planta 1982, 156, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Price, N.M.; Morel, F.M.M. Colimitation of phytoplankton growth by nickel and nitrogen. Limnol. Oceanogr. 1991, 36, 1071–1077. [Google Scholar] [CrossRef]
- Allen, A.E.; Dupont, C.L.; Oborník, M.; Horák, A.; Nunes-Nesi, A.; McCrow, J.P.; Zheng, H.; Johnson, D.A.; Hu, H.; Fernie, A.R.; et al. Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature 2011, 473, 203–207. [Google Scholar] [CrossRef] [PubMed]






| 108 | Cd | Pb | Cu | Ni | Co | T | S | pH | O2 | PO43− | NO3− | NO2− | NH4+ | SiO44− | Chl a | DOC | POC | SAS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | 0.182 | 0.433 | 0.408 | −0.311 | 0.218 | 0.632 | −0.589 | 0.147 | −0.241 | 0.331 | −0.611 | −0.690 | 0.205 | −0.556 | −0.488 | 0.734 | 0.204 | 0.189 |
| Cd | 0.391 | 0.593 | 0.581 | 0.454 | 0.037 | −0.517 | 0.384 | 0.204 | 0.440 | 0.207 | 0.014 | 0.082 | −0.279 | 0.188 | 0.395 | 0.520 | 0.228 | |
| Pb | 0.504 | 0.210 | 0.281 | 0.200 | −0.360 | 0.402 | 0.186 | 0.765 | −0.174 | −0.260 | −0.012 | −0.389 | −0.270 | 0.445 | 0.232 | 0.363 | ||
| Cu | 0.430 | 0.732 | 0.208 | −0.662 | 0.433 | 0.250 | 0.338 | −0.156 | −0.263 | 0.376 | −0.582 | 0.146 | 0.660 | 0.731 | 0.531 | |||
| Ni | 0.519 | −0.535 | −0.259 | 0.578 | 0.696 | 0.398 | 0.647 | 0.526 | 0.330 | 0.029 | 0.626 | 0.078 | 0.673 | 0.375 | ||||
| Co | 0.119 | −0.647 | 0.450 | 0.361 | 0.392 | −0.163 | −0.287 | 0.503 | −0.663 | 0.195 | 0.640 | 0.756 | 0.546 | |||||
| T | −0.471 | −0.330 | −0.737 | 0.125 | −0.687 | −0.813 | −0.101 | −0.308 | −0.467 | 0.615 | −0.046 | 0.238 | ||||||
| S | −0.564 | −0.068 | −0.444 | 0.281 | 0.443 | −0.457 | 0.591 | −0.011 | −0.900 | −0.694 | −0.503 | |||||||
| pH | 0.746 | 0.634 | 0.167 | 0.177 | 0.360 | −0.367 | 0.263 | 0.408 | 0.691 | 0.324 | ||||||||
| O2 | 0.405 | 0.420 | 0.490 | 0.459 | −0.107 | 0.482 | −0.063 | 0.487 | 0.116 | |||||||||
| PO43− | 0.102 | −0.174 | −0.209 | −0.305 | 0.286 | 0.398 | 0.538 | 0.582 | ||||||||||
| NO3− | 0.848 | −0.121 | 0.551 | 0.782 | −0.517 | 0.117 | 0.091 | |||||||||||
| NO2− | −0.144 | 0.634 | 0.649 | −0.677 | 0.039 | −0.149 | ||||||||||||
| NH4+ | −0.365 | 0.150 | 0.397 | 0.422 | 0.036 | |||||||||||||
| SiO44− | 0.314 | −0.668 | −0.416 | −0.197 | ||||||||||||||
| Chl a | −0.219 | 0.437 | 0.343 | |||||||||||||||
| DOC | 0.609 | 0.550 | ||||||||||||||||
| POC | 0.660 | |||||||||||||||||
| 107 | Cd | Pb | Cu | Ni | Co | T | S | pH | O2 | PO43− | NO3− | NO2− | NH4+ | SiO44− | Chl a | DOC | POC | SAS |
| Zn | 0.471 | 0.443 | 0.103 | −0.031 | −0.049 | 0.009 | −0.141 | −0.171 | 0.059 | −0.025 | −0.157 | −0.091 | 0.434 | −0.150 | −0.367 | 0.465 | 0.214 | 0.401 |
| Cd | 0.629 | 0.558 | 0.507 | 0.423 | −0.063 | −0.332 | 0.117 | 0.161 | 0.241 | −0.228 | 0.033 | 0.097 | 0.101 | −0.203 | 0.349 | 0.188 | 0.534 | |
| Pb | 0.364 | 0.170 | −0.077 | 0.187 | −0.199 | −0.279 | 0.024 | −0.018 | −0.524 | −0.298 | 0.321 | −0.376 | −0.464 | 0.444 | 0.165 | 0.437 | ||
| Cu | 0.591 | 0.353 | −0.127 | −0.025 | 0.163 | 0.067 | 0.274 | 0.110 | 0.119 | −0.161 | 0.138 | 0.041 | 0.097 | 0.054 | 0.161 | |||
| Ni | 0.644 | −0.618 | 0.221 | 0.642 | 0.610 | 0.390 | 0.364 | 0.513 | −0.556 | 0.338 | 0.528 | −0.254 | 0.165 | 0.046 | ||||
| Co | −0.277 | −0.198 | 0.359 | 0.296 | 0.409 | 0.088 | 0.260 | −0.171 | 0.274 | 0.217 | 0.041 | 0.190 | 0.367 | |||||
| T | −0.679 | −0.863 | −0.937 | −0.066 | −0.572 | −0.727 | 0.645 | −0.460 | −0.719 | 0.685 | −0.019 | 0.338 | ||||||
| S | 0.636 | 0.548 | −0.587 | 0.448 | 0.536 | −0.556 | 0.153 | 0.551 | −0.791 | −0.306 | −0.626 | |||||||
| pH | 0.777 | 0.103 | 0.634 | 0.777 | −0.704 | 0.517 | 0.833 | −0.718 | 0.038 | −0.365 | ||||||||
| O2 | 0.156 | 0.424 | 0.675 | −0.576 | 0.426 | 0.607 | −0.562 | 0.111 | −0.220 | |||||||||
| PO43− | 0.253 | 0.090 | −0.230 | 0.145 | 0.505 | 0.407 | 0.400 | 0.291 | ||||||||||
| NO3− | 0.688 | −0.555 | 0.574 | 0.791 | −0.561 | 0.045 | −0.385 | |||||||||||
| NO2− | −0.674 | 0.775 | 0.680 | −0.708 | −0.297 | −0.320 | ||||||||||||
| NH4+ | −0.550 | −0.741 | 0.733 | 0.196 | 0.605 | |||||||||||||
| SiO44− | 0.432 | −0.464 | −0.336 | −0.221 | ||||||||||||||
| Chl a | −0.677 | 0.194 | −0.428 | |||||||||||||||
| DOC | 0.401 | 0.635 | ||||||||||||||||
| POC | 0.259 |
| Frequency of Nutrient Limitation (%) | ||||
|---|---|---|---|---|
| 2008–2010 n = 51 | 2016–2017 n = 24 | 2016–2017 n = 27 | ||
| Western NA (101) | Eastern NA (107) | Western NA (108) | Eastern NA (107) | |
| [28]; 0–10 m Depth | This Study | |||
| P limitation PO43− < 0.1 µmol L−1 | 88 | 100 | 88 | 85 |
| N limitation TIN (NO3− + NO2− + NH4+) < 1 µmol L−1 | 24 | 33 | 46 | 67 |
| Si limitation 1 SiO44− < 2 µmol L−1 | 271 | 491 | 50 | 52 |
| Site | Dissolved TM Concentrations (nM) | Reference | |||||
|---|---|---|---|---|---|---|---|
| Zn | Cd | Pb | Cu | Ni | Co | ||
| N Adriatic/Station 108 | 5–116 | 0.04–0.18 | 0.05–0.63 | 3–17 | 4–11 | 0.19–1.22 | This study |
| N Adriatic/Station 107 | 6–224 | 0.03–0.16 | 0.05–1.25 | 3–17 | 4–19 | 0.14–0.66 | This study |
| N Adriatic/Po River | 101 ± 138 | 0.98 ± 1.07 | 1.3 ± 1 | 32 ± 8 | 35 ± 19 | 0.7 ± 0.4 | [55] |
| N Adriatic/Po plume | 0.12 ± 0.04 | 0.34 ± 0.2 | 5 ± 2 | [26] | |||
| N Adriatic | 4 | 0.08 | 7 | 9 | [56] | ||
| Mid Adriatic/Šibenik Bay | 7–101 | 0.05–0.21 | 0.05–1.11 | 6–69 | 5–11 | 0.26–0.51 | [37] |
| Mid Adriatic/Zlarin | 4 | 0.07 | 0.05 | 3 | 7 | 0.36 | [37] |
| S Adriatic | 5–10 | 0.06–0.07 | 0.05–0.14 | 2–3 | 5–9 | 0.22–0.65 | [57] |
| S Adriatic | 1–3 | 0.06–0.07 | 0.10–0.14 | 3–4 | [36] | ||
| S Adriatic | 3 | 0.08 | 3 | 5 | [56] | ||
| N Mediterranean/S France | 14–31 | 0.05–0.08 | 0.14–0.38 | 2–4 | 6–10 | 0.22–0.99 | [58] |
| W Mediterranean | 3 | 0.08 | 0.14 | 2 | 3 | [59] | |
| Sicilian Strait | 16 | 0.06 | 0.14 | 2 | 3 | [59] | |
| S Mediterranean/Sfax (Tunisia) | 190–690 | 0.14–2.21 | 0.53–2.60 | 9–21 | 14–1029 | 0.88–4.75 | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penezić, A.; Gašparović, B.; Cuculić, V.; Strmečki, S.; Djakovac, T.; Mlakar, M. Dissolved Trace Metals and Organic Matter Distribution in the Northern Adriatic, an Increasingly Oligotrophic Shallow Sea. Water 2022, 14, 349. https://doi.org/10.3390/w14030349
Penezić A, Gašparović B, Cuculić V, Strmečki S, Djakovac T, Mlakar M. Dissolved Trace Metals and Organic Matter Distribution in the Northern Adriatic, an Increasingly Oligotrophic Shallow Sea. Water. 2022; 14(3):349. https://doi.org/10.3390/w14030349
Chicago/Turabian StylePenezić, Abra, Blaženka Gašparović, Vlado Cuculić, Slađana Strmečki, Tamara Djakovac, and Marina Mlakar. 2022. "Dissolved Trace Metals and Organic Matter Distribution in the Northern Adriatic, an Increasingly Oligotrophic Shallow Sea" Water 14, no. 3: 349. https://doi.org/10.3390/w14030349