Vaccination with Recombinant Subolesin Antigens Provides Cross-Tick Species Protection in Bos indicus and Crossbred Cattle in Uganda
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cattle
2.3. Ticks
2.4. Cloning of SUB-Coding Genes and Sequence Analysis
2.5. Production of Recombinant SUB Antigens and Vaccine Formulation
2.6. Western Blot Analysis of Recombinant SUB Antigens
2.7. Vaccination Trials
2.8. Data Collection and Analysis of Vaccine Efficacy
2.9. Characterization of the Antibody Response in Vaccinated Calves
3. Results and Discussion
3.1. Experimental Design and Rationale
3.2. SUB Protein Sequences Are Highly Conserved but Show Distinctive Amino Acid Residues
3.3. The Antibody Response Was Higher in All Vaccinated Cattle when Compared to Adjuvant-Alone Treated Controls
3.4. Vaccination Affected Multiple Tick Developmental Stages with Differences between SUB Antigens, Tick Species and Cattle Breeds
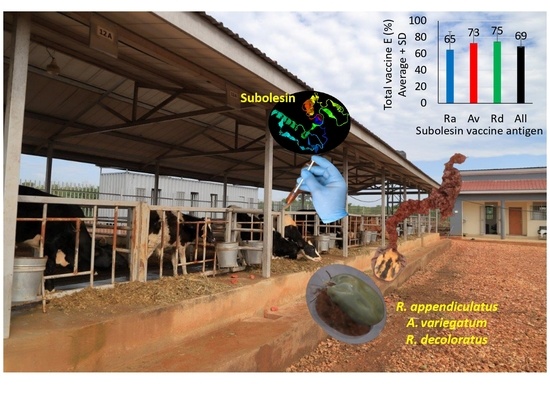
3.5. The Results of Vaccine E Support the Possibility of Using SUB Antigens for the Control of Multiple Tick Species in Different Cattle Breeds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Estrada-Peña, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhanguzi, D.; Byaruhanga, J.; Amanyire, W.; Ndekezi, C.; Ochwo, S.; Nkamwesiga, J.; Mwiine, F.N.; Tweyongyere, R.; Fourie, J.; Madder, M.; et al. Invasive cattle ticks in East Africa: Morphological and molecular confirmation of the presence of Rhipicephalus microplus in south-eastern Uganda. Parasites Vectors 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fuente, J.; Contreras, M.; Kasaija, P.D.; Gortazar, C.; Ruiz-Fons, J.F.; Mateo, R.; Kabi, F. Towards a multidisciplinary approach to improve cattle health and production in Uganda. Vaccines 2019, 7, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapholi, N.O.; Marufu, M.C.; Maiwashe, A.; Banga, C.B.; Muchenje, V.; MacNeil, M.D.; Chimonyo, M.; Dzama, K. Towards a genomics approach to tick (Acari: Ixodidae) control in cattle: A review. Ticks Tick-Borne Dis. 2014, 5, 475–483. [Google Scholar] [CrossRef]
- de la Fuente, J. Controlling ticks and tick-borne diseases…looking forward. Ticks Tick-Borne Dis. 2018, 9, 1354–1357. [Google Scholar] [CrossRef]
- de la Fuente, J.; Estrada-Peña, A. Why new vaccines for the control of ectoparasite vectors have not been registered and commercialized? Vaccines 2019, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Groot, M.J.; Van’t Hooft, K.E. The hidden effects of dairy farming on public and environmental health in the Netherlands, India, Ethiopia, and Uganda, considering the use of antibiotics and other agro-chemicals. Front. Public Health 2016, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, J.; Almazán, C.; Canales, M.; Pérez de la Lastra, J.M.; Kocan, K.M.; Willadsen, P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health Res. Rev. 2007, 8, 23–28. [Google Scholar] [CrossRef]
- Almazán, C.; Tipacamu, G.A.; Rodriguez, S.; Mosqueda, J.; Perez de Leon, A. Immunological control of ticks and tick-borne diseases that impact cattle health and production. Front. Biosci. (Landmark Ed.) 2018, 23, 1535–1551. [Google Scholar] [CrossRef] [Green Version]
- Stutzer, C.; Richards, S.A.; Ferreira, M.; Baron, S.; Maritz-Olivier, C. Metazoan parasite vaccines: Present status and future prospects. Front. Cell. Infect. Microbiol. 2018, 8, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willadsen, P. Vaccination against ectoparasites. Parasitology 2006, 133, S9–S25. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Contreras, M. Tick vaccines: Current status and future directions. Expert Rev. Vaccines 2015, 14, 1367–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fuente, J.; Kopáček, P.; Lew-Tabor, A.; Maritz-Olivier, C. Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 2016, 38, 754–769. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Szabó, M.; Labruna, M.; Mosqueda, J.; Merino, O.; Tarragona, E.; Venzal, J.M.; de la Fuente, J. Towards an effective, rational and sustainable approach for the control of cattle ticks in the Neotropics. Vaccines 2020, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Schetters, T.; Bishop, R.; Crampton, M.; Kopáček, P.; Lew-Tabor, A.; Maritz-Olivier, C.; Miller, R.; Mosqueda, J.; Patarroyo, J.; Rodriguez-Valle, M.; et al. Cattle tick vaccine researchers join forces in CATVAC. Parasites Vectors 2016, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Artigas-Jerónimo, S.; Villar, M.; Cabezas-Cruz, A.; Valdés, J.J.; Estrada-Peña, A.; Alberdi, P.; de la Fuente, J. Functional evolution of Subolesin/Akirin. Front. Physiol. 2018, 9, 1612. [Google Scholar] [CrossRef]
- Adaszek, L.; Winiarczyk, S. Molecular characterization of Babesia canis canis isolates from naturally infected dogs in Poland. Vet. Parasitol. 2008, 152, 235–241. [Google Scholar] [CrossRef]
- Chaligiannis, Ι.; Fernández de Mera, I.G.; Papa, A.; Sotiraki, S.; de la Fuente, J. Molecular identification of tick-borne pathogens in ticks collected from dogs and small ruminants from Greece. Exp. Appl. Acarol. 2018, 74, 443–453. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Almazán, C.; Moreno-Cantú, O.; Moreno-Cid, J.A.; Galindo, R.C.; Canales, M.; Villar, M.; de la Fuente, J. Control of tick infestations in cattle vaccinated with bacterial membranes containing surface-exposed tick protective antigens. Vaccine 2012, 30, 265–272. [Google Scholar] [CrossRef]
- Rodríguez, M.; Massard, C.L.; Henrique da Fonseca, A.; Fonseca Ramos, N.; Machado, H.; Labarta, V.; de la Fuente, J. Effect of vaccination with a recombinant Bm86 antigen preparation on natural infestations of Boophilus microplus in grazing dairy and beef pure and cross-bred cattle in Brazil. Vaccine 1995, 13, 1804–1808. [Google Scholar] [CrossRef]
- Aguirre Ade, A.; Garcia, M.V.; Szabó, M.P.; Barros, J.C.; Andreotti, R. Formula to evaluate efficacy of vaccines and systemic substances against three-host ticks. Int. J. Parasitol. 2015, 45, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, M.; Villar, M.; de la Fuente, J. A vaccinomics approach to the identification of tick protective antigens for the control of Ixodes ricinus and Dermacentor reticulatus infestations in companion animals. Front. Physiol. 2019, 10, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, M.; Moreno-Cid, J.A.; Domingos, A.; Canales, M.; Díez-Delgado, I.; Pérez de la Lastra, J.M.; Sánchez, E.; Merino, O.; López Zavala, R.; Ayllón, N.; et al. Bacterial membranes enhance the immunogenicity and protective capacity of the surface exposed tick Subolesin-Anaplasma marginale MSP1a chimeric antigen. Ticks Tick-Borne Dis. 2015, 6, 820–828. [Google Scholar] [CrossRef]
- de la Fuente, J.; Moreno-Cid, J.A.; Galindo, R.C.; Almazán, C.; Kocan, K.M.; Merino, O.; Pérez de la Lastra, J.M.; Estrada-Peña, A.; Blouin, E.F. Subolesin/Akirin vaccines for the control of arthropod vectors and vector-borne pathogens. Transbound. Emerg. Dis. 2013, 60, 172–178. [Google Scholar] [CrossRef]
- Moreno-Cid, J.A.; Pérez de la Lastra, J.M.; Villar, M.; Jiménez, M.; Pinal, R.; Estrada-Peña, A.; Alarcón, P.; Delacour, S.; Oropeza, V.; Ruiz, I.; et al. Control of multiple arthropod vector infestations with subolesin/akirin vaccines. Vaccine 2013, 31, 1187–1196. [Google Scholar] [CrossRef]
- Macqueen, D.J.; Johnston, I.A. Evolution of the multifaceted eukaryotic akirin gene family. BMC Evol. Biol. 2009, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Galindo, R.C.; Muñoz, P.M.; de Miguel, M.J.; Marin, C.M.; Blasco, J.M.; Gortazar, C.; Kocan, K.M.; de la Fuente, J. Differential expression of inflammatory and immune response genes in rams experimentally infected with a rough virulent strain of Brucella ovis. Vet. Immunol. Immunopathol. 2009, 127, 295–303. [Google Scholar] [CrossRef]
- Sultana, H.; Patel, U.; Sonenshine, D.E.; Neelakanta, G. Identification and comparative analysis of subolesin/akirin ortholog from Ornithodoros turicata ticks. Parasites Vectors 2015, 8, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artigas-Jerónimo, S.; Pastor Comín, J.J.; Villar, M.; Contreras, M.; Alberdi, P.; León Viera, I.; Soto, L.; Cordero, R.; Valdés, J.J.; Cabezas-Cruz, A.; et al. A novel combined scientific and artistic approach for advanced characterization of interactomes: The Akirin/Subolesin model. Vaccines 2020, 8, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fuente, J.; Moreno-Cid, J.A.; Canales, M.; Villar, M.; Pérez de la Lastra, J.M.; Kocan, K.M.; Galindo, R.C.; Almazán, C.; Blouin, E.F. Targeting arthropod subolesin/akirin for the development of a universal vaccine for control of vector infestations and pathogen transmission. Vet. Parasitol. 2011, 181, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Kasaija, P.D.; Merino, O.; de la Cruz-Hernandez, N.I.; Gortazar, C.; de la Fuente, J. Oral vaccination with a formulation combining Rhipicephalus microplus Subolesin with heat inactivated Mycobacterium bovis reduces tick infestations in cattle. Front. Cell. Infect. Microbiol. 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Merino, M.; Antunes, S.; Mosqueda, J.; Moreno-Cid, J.A.; Pérez de la Lastra, J.M.; Rosario-Cruz, R.; Rodríguez, S.; Domingos, A.; de la Fuente, J. Vaccination with proteins involved in tick-pathogen interactions reduces vector infestations and pathogen infection. Vaccine 2013, 31, 5889–5896. [Google Scholar] [CrossRef]
- Shakya, M.; Kumar, B.; Nagar, G.; de la Fuente, J.; Ghosh, S. Subolesin: A candidate vaccine antigen for the control of cattle tick infestations in Indian situation. Vaccine 2014, 32, 3488–3494. [Google Scholar] [CrossRef]
- Slifka, M.K.; Amanna, I. How advances in immunology provide insight into improving vaccine efficacy. Vaccine 2014, 32, 2948–2957. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Frego, L.; Lasaro, M.; Truncali, K.; Kroe-Barrett, R.; Singh, S. Efficient Qualitative and Quantitative Determination of Antigen-induced Immune Responses. J. Biol. Chem. 2016, 291, 16361–16374. [Google Scholar] [CrossRef] [Green Version]
- Prechl, J. A generalized quantitative antibody homeostasis model: Antigen saturation, natural antibodies and a quantitative antibody network. Clin. Transl. Immunol. 2017, 6, e131. [Google Scholar] [CrossRef]
- Canales, M.; Enríquez, A.; Ramos, E.; Cabrera, D.; Dandie, H.; Soto, A.; Falcón, V.; Rodríguez, M.; de la Fuente, J. Large-scale production in Pichia pastoris of the recombinant vaccine Gavac against cattle tick. Vaccine 1997, 15, 414–422. [Google Scholar] [CrossRef]
- Popara, M.; Villar, M.; Mateos-Hernández, L.; de Mera, I.G.; Marina, A.; del Valle, M.; Almazán, C.; Domingos, A.; de la Fuente, J. Lesser protein degradation machinery correlates with higher BM86 tick vaccine efficacy in Rhipicephalus annulatus when compared to Rhipicephalus microplus. Vaccine 2013, 31, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Olds, C.L.; Mwaura, S.; Odongo, D.O.; Scoles, G.A.; Bishop, R.; Daubenberger, C. Induction of humoral immune response to multiple recombinant Rhipicephalus appendiculatus antigens and their effect on tick feeding success and pathogen transmission. Parasites Vectors 2016, 9, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobon, G.; Hungerford, J.; Woodrow, M.; Smith, D.; Willadsen, P. Vaccination against Boophilus microplus: The Australian field experience. In Recombinant Vaccines for the Control of Cattle Tick; de la Fuente, J., Ed.; Elfos Scientiae: Havana, Cuba, 1995; pp. 163–176. [Google Scholar]
- de la Fuente, J.; Rodríguez, M.; Redondo, M.; Montero, C.; García-García, J.C.; Méndez, L.; Serrano, E.; Valdés, M.; Enríquez, A.; Canales, M.; et al. Field studies and cost-effectiveness analysis of vaccination with GavacTM against the cattle tick Boophilus microplus. Vaccine 1998, 16, 366–373. [Google Scholar] [CrossRef]
- Contreras, M.; de la Fuente, J. Control of Ixodes ricinus and Dermacentor reticulatus tick infestations in rabbits vaccinated with the Q38 Subolesin/Akirin chimera. Vaccine 2016, 34, 3010–3013. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Singh, N.K.; Das, G. Assessment of duration of immunity in crossbred cattle immunized with glycoproteins isolated from Hyalomma anatolicum anatolicum and Boophilus microplus. Parasitol. Res. 2005, 95, 319–326. [Google Scholar] [CrossRef]
- Rego, R.O.M.; Trentelman, J.J.A.; Anguita, J.; Nijhof, A.M.; Sprong, H.; Klempa, B.; Hajdusek, O.; Tomás-Cortázar, J.; Azagi, T.; Strnad, M.; et al. Counterattacking the tick bite: Towards a rational design of anti-tick vaccines targeting pathogen transmission. Parasites Vectors 2019, 12, 229. [Google Scholar] [CrossRef]







| Vaccination with R. appendiculatus SUB | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | DL | DMn | DN | DMa | DA | DO | DF | E |
| B. indicus | ||||||||
| R. appendiculatus | 17% * | 22% * | 13% * | 0% | 19% | 6% * | 0% | 47% |
| A. variegatum | 0% | 25% * | 0% | 3% * | 19% | 31% * | 0% | 50% |
| Crossbred | ||||||||
| R. appendiculatus | 41% * | 64% * | 0% | 0% | 51% * | 0% | 0% | 90% |
| A. variegatum | 34% * | 44% * | 19% * | 25% * | 0% | 4% * | 49% * | 89% |
| R. decoloratus | 0% | 8% * | 47% * | 51% | ||||
| Vaccination with A. variegatum SUB | ||||||||
| Group | DL | DMn | DN | DMa | DA | DO | DF | E |
| B. indicus | ||||||||
| R. appendiculatus | 61% * | 51% * | 0% | 0% | 29% * | 0% | 0% | 86% |
| A. variegatum | 0% | 1% * | 7% * | 3% * | 41%* | 0% | 0% | 47% |
| Crossbred | ||||||||
| R. appendiculatus | 53% * | 39% * | 0% | 39% * | 0% | 4% * | 0% | 83% |
| A. variegatum | 54% * | 28% * | 16% * | 0% | 0% | 14% * | 0% | 76% |
| R. decoloratus | 0% | 0% | 72% * | 72% | ||||
| Vaccination with R. decoloratus SUB | ||||||||
| Group | DL | DMn | DN | DMa | DA | DO | DF | E |
| B. indicus | ||||||||
| R. appendiculatus | 0% | 0% | 25% * | 0% | 55% * | 0% | 0% | 66% |
| A. variegatum | 0% | 0% | 32% * | 0% | 38% * | 0% | 0% | 58% |
| Crossbred | ||||||||
| R. appendiculatus | 62% * | 47% * | 25% * | 19% * | 0% | 10% * | 0% | 89% |
| A. variegatum | 69% * | 50% * | 19% * | 18% * | 0% | 0% | 39% * | 94% |
| R. decoloratus | 0% | 0% | 69%* | 69% | ||||
| Vaccination with the combination of SUB antigens | ||||||||
| Group | DL | DMn | DN | DMa | DA | DO | DF | E |
| B. indicus | ||||||||
| R. appendiculatus | 38% * | 53% * | 0% | 0% | 57% * | 0% | 38% * | 92% |
| A. variegatum | 0% | 0% | 0% | 0% | 51%* | 0% | 0% | 51% |
| Crossbred | ||||||||
| R. appendiculatus | 29% * | 53% * | 0% | 22% * | 0% | 0% | 0% | 74% |
| A. variegatum | 27% * | 0% | 7% * | 28% * | 0% | 13% * | 0% | 69% |
| R. decoloratus | 0% | 0% | 71% * | 71% | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasaija, P.D.; Contreras, M.; Kabi, F.; Mugerwa, S.; de la Fuente, J. Vaccination with Recombinant Subolesin Antigens Provides Cross-Tick Species Protection in Bos indicus and Crossbred Cattle in Uganda. Vaccines 2020, 8, 319. https://doi.org/10.3390/vaccines8020319
Kasaija PD, Contreras M, Kabi F, Mugerwa S, de la Fuente J. Vaccination with Recombinant Subolesin Antigens Provides Cross-Tick Species Protection in Bos indicus and Crossbred Cattle in Uganda. Vaccines. 2020; 8(2):319. https://doi.org/10.3390/vaccines8020319
Chicago/Turabian StyleKasaija, Paul D., Marinela Contreras, Fredrick Kabi, Swidiq Mugerwa, and José de la Fuente. 2020. "Vaccination with Recombinant Subolesin Antigens Provides Cross-Tick Species Protection in Bos indicus and Crossbred Cattle in Uganda" Vaccines 8, no. 2: 319. https://doi.org/10.3390/vaccines8020319