Thermodynamic Analysis and Experimental Study of Selective Dehydrogenation of 1,2-cyclohexanediol over Cu2+1O/MgO Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cu/MgO Catalyst
2.2. Catalyst Characterization
2.3. Evaluation of Catalytic Activity
2.4. Calculation of Thermodynamics
3. Results and Discussion
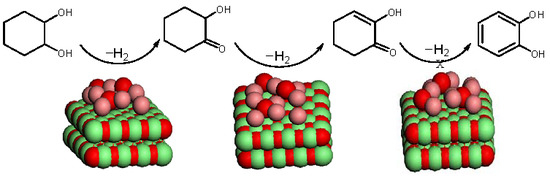
3.1. Thermodynamic Analysis
3.1.1. Stability Analysis of Primary Dehydrogenation Products
3.1.2. Thermodynamic Analysis of the Dehydrogenation Reaction in the Gas Phase

3.2. Catalyst Characterization Results
3.3. Catalytic Performance of Cu/MgO in the Dehydrogenation of CHD
3.3.1. Effects of Preparation Conditions on the Catalytic Performance of Cu/MgO
3.3.2. Effect of Reaction Conditions on the Dehydrogenation of CHD
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, G.; Dong, Y.; Jiang, L.; He, D.; Yang, Y.; Zhou, X. Effect of support composition on the structural and catalytic properties of Ru/AlOOH-SiO2 catalysts for benzene selective hydrogenation. Catal. Sci. Technol. 2018, 8, 1435–1446. [Google Scholar] [CrossRef]
- Spod, H.; Lucas, M.; Claus, P. Selective hydrogenation of benzene to cyclohexene over 2Ru/La2O3-ZnO catalyst without additional modifiers. ChemCatChem 2016, 8, 2659–2666. [Google Scholar] [CrossRef]
- Liu, J.; Xu, S.; Bing, W.; Wang, F.; Li, C.; Wei, M.; Evans, D.G.; Duan, X. Cu-decorated Ru catalysts supported on layered double hydroxides for selective benzene hydrogenation to cyclohexene. ChemCatChem 2015, 7, 846–855. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Q.; Zhu, M.; Wang, Z. Selective hydrogenation of benzene to cyclohexene over Ru-Zn/ZrO2 catalysts prepared by a two-step impregnation method. J. Mol. Catal. A Chem. 2016, 413, 85–93. [Google Scholar] [CrossRef]
- Alimardanov, K.M.; Sadygov, O.A.; Garibov, N.I.; Abbasov, M.F.; Abdullaeva, M.Y.; Dzhafarova, N.A. Liquid-phase catalytic oxidation of C6-C7 cycloolefins into carboxylic acids in a pseudohomogeneous system. Russ. J. Appl. Chem. 2011, 84, 236–242. [Google Scholar] [CrossRef]
- Saha, D.; Hazra, D.K.; Maity, T.; Koner, S. Heterometallic metal-organic frameworks that catalyze two different reactions sequentially. Inorg. Chem. 2016, 55, 5729–5731. [Google Scholar] [CrossRef] [PubMed]
- Damm, M.; Gutmann, B.; Kappe, C.O. Continuous-flow synthesis of adipic acid from cyclohexene using hydrogen peroxide in high-temperature explosive regimes. ChemSusChem 2013, 6, 978–982. [Google Scholar] [CrossRef]
- Chen, B.H.; Liu, W.; Li, A.; Liu, Y.J.; Chao, Z.S. A simple and convenient approach for preparing core-shell-like silica@nickel species nanoparticles: Highly efficient and stable catalyst for the dehydrogenation of 1,2-cyclohexanediol to catechol. Dalton T. 2015, 44, 1023–1038. [Google Scholar] [CrossRef]
- Paushkin, Y.M.; Nizova, S.A.; Stytsenko, V.D.; Belov, P.S.; Rozovskii, A.Y.; D’Yakonov, A.Y. Preparation of pyrocatechol and its alkyl and aryl derivatives. Dokl. Chem. 1983, 271, 217–219. [Google Scholar]
- Zhou, Z.Z.; Liu, M.; Lv, L.; Li, C.J. Silver(I)-catalyzed widely applicable aerobic 1,2-diol oxidative cleavage. Angew. Chem. Int. Edit. 2018, 57, 2616–2620. [Google Scholar] [CrossRef]
- Ghosh, S.; Acharyya, S.S.; Adak, S.; Konathala, L.N.S.; Sasaki, T.; Bal, R. Selective oxidation of cyclohexene to adipic acid over silver supported tungsten oxide nanostructured catalysts. Green Chem. 2014, 16, 2826–2834. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, J.; Xing, J.; Tang, S.F.; Song, L.; Sun, Y.; Zhang, C.; Xin, H.; Li, X. An investigation on the aqueous-phase hydrodeoxygenation of various methoxy-substituted lignin monomers on Pd/C and HZSM-5 catalysts. RSC. Adv. 2016, 6, 104398–104406. [Google Scholar] [CrossRef]
- Cho, C.S.; Oh, S.G. A new ruthenium-catalyzed approach for quinoxalines from ortho-phenylenediamines and vicinal-diols. Tetrahedron Lett. 2006, 47, 5633–5636. [Google Scholar] [CrossRef]
- Lee, H.; Yi, C.S. Catalytic synthesis of substituted indoles and quinolines from the dehydrative C-H coupling of arylamines with 1,2- and 1,3-Diols. Organometallics 2016, 35, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Zhang, S.D.; Chen, L.; Li, B.; Jiang, H.F.; Zhang, M. An annulative transfer hydrogenation strategy enables straightforward access to tetrahydro fused-pyrazine derivatives. Chem. Commun. 2016, 52, 10636–10639. [Google Scholar] [CrossRef] [PubMed]
- Bierenstiel, M.; D’Hondt, P.J.; Schlaf, M. Investigations into the selective oxidation of vicinal diols to α-hydroxy ketones with the NaBrO3/NaHSO3 reagent: pH dependence, stoichiometry, substrates and origin of selectivity. Tetrahedron 2005, 61, 4911–4917. [Google Scholar] [CrossRef]
- William, J.M.; Kuriyama, M.; Onomura, O. An efficient method for selective oxidation of 1,2-Diols in water catalyzed by Me2SnCl2. RSC. Adv. 2013, 3, 19247–19250. [Google Scholar] [CrossRef]
- Obara, N.; Hirasawa, S.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Oxidative cleavage of vicinal diols with the combination of platinum and vanadium catalysts and molecular oxygen. ChemCatChem 2016, 8, 1732–1738. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Liu, S.T. Polymer supported vanadium complexes as catalysts for the oxidation of alkenes in water. Catal. Lett. 2010, 139, 61–66. [Google Scholar] [CrossRef]
- Verma, S.; Jain, S.L.; Sain, B. Poly(ethylene glycol) embedded potassium tribromide (PEG•KBr3) as a recyclable catalyst for oxidation of alcohols. Ind. Eng. Chem. Res. 2011, 50, 5862–5865. [Google Scholar] [CrossRef]
- Khenkin, A.M.; Neumann, R. Aerobic oxidation of vicinal diols catalyzed by an anderson-type polyoxometalate, [IMo6O24]5−. Adv. Synth. Catal. 2002, 344, 1017–1021. [Google Scholar] [CrossRef]
- Sato, H.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Synthesis of alpha-hydroxy ketones from vicinal diols by selective dehydrogenation over Ir-ReOX/SiO2 catalyst. Chem. Lett. 2014, 43, 334–336. [Google Scholar] [CrossRef]
- Zhou, G.D.; Guo, X.H.; Lu, X.J.; Li, Y.N.; Cheng, T.X.; Li, W.X.; Zhen, K.J. MgO-Supported Cu2PMo11VO40 Catalyst for Oxidative Dehydrogenation of n-Hexanol to n-Hexanal. Chin. J. Catal. 2005, 26, 389–392. [Google Scholar]
- Ji, D.; Zhu, W.; Wang, Z.; Wang, G. Dehydrogenation of cyclohexanol on Cu-ZnO/SiO2 catalysts: The role of copper species. Catal. Commun. 2007, 8, 1891–1895. [Google Scholar] [CrossRef]
- Fridman, V.Z.; Davydov, A.A.; Titievsky, K. Dehydrogenation of cyclohexanol on copper-containing catalysts: II. The pathways of the cyclohexanol dehydrogenation reaction to cyclohexanone on copper-active sites in oxidation state Cu0 and Cu+. J. Catal. 2004, 222, 545–557. [Google Scholar] [CrossRef]
- Fridman, V.Z.; Davydov, A.A. Dehydrogenation of cyclohexanol on copper-containing catalysts: I. The influence of the oxidation state of copper on the activity of copper sites. J. Catal. 2000, 195, 20–30. [Google Scholar] [CrossRef]
- Luna, A.; Alcamıí, M.; Mó, O.; Máñez, M. Cu+ reactivity trends in sp, sp2, and sp3 nitrogen, phosphorus, and arsenic containing bases. Int. J. Mass. Spectrom 2000, 201, 215–231. [Google Scholar] [CrossRef]
- Trost, B.M.; Schroeder, G.M. Cyclic 1,2-diketones as building blocks for asymmetric synthesis of cycloalkenones. J. Am. Chem. Soc. 2000, 122, 3785–3786. [Google Scholar] [CrossRef]
- Svennebring, A.; Nilsson, P.; Larhed, M. Microwave-accelerated spiro-cyclizations of o-halobenzyl cyclohexenyl ethers by palladium (0) catalysis. J. Org. Chem. 2007, 72, 5851–5854. [Google Scholar] [CrossRef]
- Jeena, V.; Robinson, R.S. Green oxidations: Titanium dioxide induced tandem oxidation coupling reactions. Beilstein. J. Org. Chem. 2009, 5, 24. [Google Scholar] [CrossRef]
- Araki, Y.; Miyawaki, A.; Miyashita, T.; Mizutani, M.; Hirai, N.; Todoroki, Y. A new non-azole inhibitor of ABA 8′-hydroxylase: Effect of the hydroxyl group substituted for geminal methyl groups in the six-membered ring. Bioorg. Med. Chem. Lett. 2006, 16, 3302–3305. [Google Scholar] [CrossRef]
- Cleghorn, L.A.T.; Cooper, I.R.; Fishwick, C.W.G.; Grigg, R.; MacLachlan, W.S.; Rasparini, M.; Sridharan, V. Three-component bimetallic (Pd/In) mediated cascade allylation of C=X functionality: Part 1. Scope and class 1 examples with aldehydes and ketones. J. Organomet. Chem. 2003, 687, 483–493. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2016; Available online: http://gaussian.com/g09citation/ (accessed on 9 February 2019).
- Tomasi, J.; Mennucci, B.; Cances, E. The IEF version of the PCM solvation method: an overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Xu, G.Y.; Guo, J.H.; Qu, Y.C.; Zhang, Y.; Fu, Y.; Guo, Q.X. Selective hydrodeoxygenation of lignin-derived phenols to alkyl cyclohexanols over a Ru-solid base bifunctional catalyst. Green Chem. 2016, 18, 5510–5517. [Google Scholar] [CrossRef]
- Song, W.; Liu, Y.; Baráth, E.; Zhao, C.; Lercher, J.A. Synergistic effects of Ni and acid sites for hydrogenation and C-O bond cleavage of substituted phenols. Green Chem. 2015, 17, 1204–1218. [Google Scholar] [CrossRef]
- Liu, T.; Ma, P.C.; Yu, J.K.; Li, L.; Liu, X.; Ma, B. Preparation of MgO by Thermal Decomposition of Mg(OH)2. J. Chin. Ceram. Soc. 2010, 38, 1337–1340. [Google Scholar]
- Meda, L.; Cerofolini, G.F. A decomposition procedure for the determination of copper oxidation states in Cu-zeolites by XPS. Surf. Interface Anal. 2004, 36, 756–759. [Google Scholar] [CrossRef]










| T (°C) | Primary Dehydrogenation Product (kcal/mol) | Secondary Dehydrogenation Product (kcal/mol) | ||||
|---|---|---|---|---|---|---|
 |  |  |  |  |  | |
| 25 a | 0.00 | 17.52 | 23.15 | 0.00 | 5.75 | 30.00 |
| 100 a | 0.00 | 13.89 | 18.43 | 0.00 | 4.33 | 23.84 |
| 220 a | 0.00 | 10.35 | 18.26 | 0.00 | 3.91 | 23.56 |
| 25 b | 0.00 | 18.71 | 23.72 | 0.00 | 0.91 | 30.04 |
| 25 c | 0.00 | 18.98 | 24.25 | 0.00 | 0.71 | 30.04 |
| T (°C) | ∆H (kcal/mol) | ∆G (kcal/mol) | ||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (1) | (2) | (3) | |
| 25 | 11.59 | 18.21 | 8.97 | 2.74 | 9.57 | 0.15 |
| 50 | 11.70 | 18.34 | 9.13 | 2.00 | 8.84 | −0.60 |
| 100 | 11.89 | 18.57 | 9.44 | 0.48 | 7.36 | −2.13 |
| 150 | 12.05 | 18.78 | 9.72 | −1.06 | 5.84 | −3.70 |
| 200 | 12.18 | 18.96 | 9.98 | −2.61 | 4.30 | −5.30 |
| 220 | 12.23 | 19.03 | 10.07 | −3.24 | 3.68 | −5.94 |
| 250 | 12.30 | 19.12 | 10.20 | −4.18 | 2.74 | −6.92 |
| 300 | 12.39 | 19.25 | 10.40 | −5.76 | 1.17 | −8.57 |
| Catalysts | Specific Surface Area (m2/g) | Mass Ratio of Cu to MgO a | Crystallite Sizes (nm, XRD) | |
|---|---|---|---|---|
| Cu2+1O | MgO | |||
| 0.14Cu/MgO-C-350 | 49.3 | 0.14:1 | 27.8 | 8.8 |
| 0.24Cu/MgO-C-350 | 47.4 | 0.24:1 | 29.0 | 8.7 |
| 0.30Cu/MgO-C-350 | 44.6 | 0.30:1 | 34.2 | 11.7 |
| 0.28Cu/MgO-IM-350 b | 29.9 | 0.28:1 | 35.2 | 49.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yang, Q.; Song, Y.; Wang, Y. Thermodynamic Analysis and Experimental Study of Selective Dehydrogenation of 1,2-cyclohexanediol over Cu2+1O/MgO Catalysts. Sustainability 2019, 11, 902. https://doi.org/10.3390/su11030902
Wang H, Yang Q, Song Y, Wang Y. Thermodynamic Analysis and Experimental Study of Selective Dehydrogenation of 1,2-cyclohexanediol over Cu2+1O/MgO Catalysts. Sustainability. 2019; 11(3):902. https://doi.org/10.3390/su11030902
Chicago/Turabian StyleWang, Haiou, Qiusheng Yang, Yucong Song, and Yanji Wang. 2019. "Thermodynamic Analysis and Experimental Study of Selective Dehydrogenation of 1,2-cyclohexanediol over Cu2+1O/MgO Catalysts" Sustainability 11, no. 3: 902. https://doi.org/10.3390/su11030902