Reducing Results Variance in Lifespan Machines: An Analysis of the Influence of Vibrotaxis on Wild-Type Caenorhabditis elegans for the Death Criterion
Abstract
:1. Introduction
2. Materials and Methods
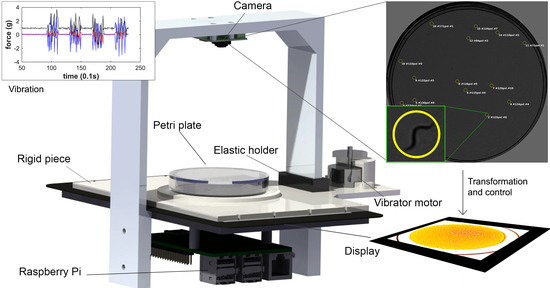
2.1. Lifespan Machine
2.2. Vibration Mechanism
2.3. Sample Design
2.4. Experimental Design
2.4.1. Methodology before and after the Vibration Conditions
2.4.2. Experiment 1: Vibration Timing Experiment
2.4.3. Experiment 2: Habituation Experiment
2.4.4. Experiment 3: Lifespan Error Experiment
2.5. Ground-Truth Data for the Validation Method
3. Results
3.1. Vibrotaxis Analysis
3.2. Habituation Analysis
3.3. Error Variability Analysis
3.4. Vibrotaxis Effect on the Lifespan Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amrit, F.R.G.; Ratnappan, R.; Keith, S.A.; Ghazi, A. The C. elegans lifespan assay toolkit. Methods 2014, 68, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.W.; McColl, G.; Jenkins, N.L.; Harris, J.; Lithgow, G.J. Evolution of lifespan in C. elegans. Nature 2000, 405, 296–297. [Google Scholar] [CrossRef] [PubMed]
- Herndon, L.A.; Schmeissner, P.J.; Dudaronek, J.M.; Brown, P.A.; Listner, K.M.; Sakano, Y.; Paupard, M.C.; Hall, D.H.; Driscoll, M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 2002, 419, 808. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.D.; Mathew, N.D.; Ebert, P.R. WormScan: A Technique for High-Throughput Phenotypic Analysis of Caenorhabditis elegans. PLoS ONE 2012, 7, e33483. [Google Scholar] [CrossRef] [Green Version]
- Stroustrup, N.; Ulmschneider, B.E.; Nash, Z.M.; López-Moyado, I.F.; Apfeld, J.; Fontana, W. The Caenorhabditis elegans Lifespan Machine. Nat. Methods 2013, 10, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Xian, B.; Shen, J.; Chen, W.; Sun, N.; Qiao, N.; Jiang, D.; Yu, T.; Men, Y.; Han, Z.; Pang, Y.; et al. WormFarm: A quantitative control and measurement device toward automated Caenorhabditis elegans aging analysis. Aging Cell 2013, 12, 398–409. [Google Scholar] [CrossRef]
- Petzold, B.C.; Park, S.J.; Mazzochette, E.A.; Goodman, M.B.; Pruitt, B.L. MEMS-based force-clamp analysis of the role of body stiffness in C. elegans touch sensation. Integr. Biol. 2013, 5, 853–864. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.A.; Wang, Q.; Hasani, R.M.; Balun, O.; Clarke, E.M.; Grosu, R.; Smolka, S.A. Probabilistic Reachability Analysis of the Tap Withdrawal Circuit in Caenorhabditis elegans. In Proceedings of the 2016 IEEE International High Level Design Validation and Test Workshop (Hldvt), Santa Cruz, CA, USA, 7–8 October 2016; pp. 170–177. [Google Scholar]
- Amano, S.; Kitamura, K.; Hosono, R. Hierarchy of habituation induced by mechanical stimuli in Caenorhabditis elegans. Zool. Sci. 1999, 16, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Wicks, S.R.; Rankin, C.H. The integration of antagonistic reflexes revealed by laser ablation of identified neurons determines habituation kinetics of the Caenorhabditis elegans tap withdrawal response. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 1996, 179, 675–685. [Google Scholar] [CrossRef]
- Wicks, S.R.; Rankin, C.H. Integration of Mechanosensory Stimuli in Caenorhabditis-Elegans. J. Neurosci. 1995, 15, 2434–2444. [Google Scholar] [CrossRef] [Green Version]
- Chalfie, M. Modulation of C. elegans Touch Sensitivity Is Integrated at Multiple Levels. J. Neurosci. 2014, 34, 6522–6536. [Google Scholar] [CrossRef]
- Childs, P.G.; Boyle, C.A.; Pemberton, G.D.; Nikukar, H.; Curtis, A.S.G.; Henriquez, F.L.; Dalby, M.J.; Reid, S. Use of nanoscale mechanical stimulation for control and manipulation of cell behaviour. ACTA Biomater. 2016, 34, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Meyhöfer, R.; Casas, J. Vibratory stimuli in host location by parasitic wasps. J. Insect Physiol. 1999, 45, 967–971. [Google Scholar] [CrossRef]
- Sugi, T.; Okumura, E.; Kiso, K.; Igarashi, R. Nanoscale Mechanical Stimulation Method for Quantifying C. elegans Mechanosensory Behavior and Memory. Anal. Sci. 2016, 32, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Chalfie, M.; Sulston, J.E.; White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The Neural Circuit for Touch Sensitivity in Caenorhabditis-Elegans. J. Neurosci. 1985, 5, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Rankin, C.H.; Beck, C.D.O.; Chiba, C.M. Caenorhabditis elegans: A new model system for the study of learning and memory. Behav. Brain Res. 1990, 37, 89–92. [Google Scholar] [CrossRef]
- Swierczek, N.A.; Giles, A.C.; Rankin, C.H.; Kerr, R.A. High-throughput behavioral analysis in C. elegans. Nat. Methods 2011, 8, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Wang, J.; Wang, K.; Huang, B.; Niu, L.; Li, F.; Cai, F.; Chen, Y.; Liu, X.; Zhang, X.; et al. Ultrasound neuro-modulation chip: Activation of sensory neurons in Caenorhabditis elegans by surface acoustic waves. Lab Chip 2017, 17, 1725–1731. [Google Scholar] [CrossRef]
- Ward, A.; Liu, J.; Feng, Z.; Xu, X.Z.S. Light-sensitive neurons and channels mediate phototaxis in C. elegans. Nat. Neurosci. 2008, 11, 916–922. [Google Scholar] [CrossRef]
- Lee, K.; Aschner, M. A Simple Light Stimulation of Caenorhabditis elegans. Curr. Protoc. Toxicol. 2016, 67, 11.21.1–11.21.5. [Google Scholar] [CrossRef] [Green Version]
- Nagel, G.; Brauner, M.; Liewald, J.F.; Adeishvili, N.; Bamberg, E.; Gottschalk, A. Light Activation of Channelrhodopsin-2 in Excitable Cells of Caenorhabditis elegans Triggers Rapid Behavioral Responses. Curr. Biol. 2005, 15, 2279–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, S.L.; Charlie, N.K.; Milfort, M.C.; Brown, B.S.; Gravlin, C.N.; Knecht, J.E.; Miller, K.G. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 2008, 6, 1715–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churgin, M.A.; Jung, S.K.; Yu, C.C.; Chen, X.; Raizen, D.M.; Fang-Yen, C. Longitudinal imaging of Caenorhabditis elegans in a microfabricated device reveals variation in behavioral decline during aging. eLife 2017, 6, e26652. [Google Scholar] [CrossRef] [PubMed]
- De Magalhaes Filho, C.D.; Henriquez, B.; Seah, N.E.; Evans, R.M.; Lapierre, L.R.; Dillin, A. Visible light reduces C. elegans longevity. Nat. Commun. 2018, 9, 927. [Google Scholar] [CrossRef] [PubMed]
- Puchalt, J.C.; Sánchez-Salmerón, A.J.; Martorell Guerola, P.; Genovés Martínez, S. Active backlight for automating visual monitoring: An analysis of a lighting control technique for Caenorhabditis elegans cultured on standard Petri plates. PLoS ONE 2019, 14, e0215548. [Google Scholar] [CrossRef]
- Puchalt, J.C.; Sánchez-Salmerón, A.J.; Ivorra, E.; Genovés Martínez, S.; Martínez, R.; Martorell Guerola, P. Improving lifespan automation for Caenorhabditis elegans by using image processing and a post-processing adaptive data filter. Sci. Rep. 2020, 10, 8729. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. WormBook 2006. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.H.; Stiles, J.W.; Santelli, J.; Sanadi, D.R. Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J. Gerontol. 1979, 34, 28–36. [Google Scholar] [CrossRef]
- Cronin, C.J.; Mendel, J.E.; Mukhtar, S.; Kim, Y.M.; Stirbl, R.C.; Bruck, J.; Sternberg, P.W. An automated system for measuring parameters of nematode sinusoidal movement. BMC Genet. 2005, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Hahm, J.H.; Kim, S.; DiLoreto, R.; Shi, C.; Lee, S.J.V.; Murphy, C.T.; Nam, H.G. C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nat. Commun. 2015, 6, 8919. [Google Scholar] [CrossRef] [Green Version]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchalt, J.C.; Layana Castro, P.E.; Sánchez-Salmerón, A.-J. Reducing Results Variance in Lifespan Machines: An Analysis of the Influence of Vibrotaxis on Wild-Type Caenorhabditis elegans for the Death Criterion. Sensors 2020, 20, 5981. https://doi.org/10.3390/s20215981
Puchalt JC, Layana Castro PE, Sánchez-Salmerón A-J. Reducing Results Variance in Lifespan Machines: An Analysis of the Influence of Vibrotaxis on Wild-Type Caenorhabditis elegans for the Death Criterion. Sensors. 2020; 20(21):5981. https://doi.org/10.3390/s20215981
Chicago/Turabian StylePuchalt, Joan Carles, Pablo E. Layana Castro, and Antonio-José Sánchez-Salmerón. 2020. "Reducing Results Variance in Lifespan Machines: An Analysis of the Influence of Vibrotaxis on Wild-Type Caenorhabditis elegans for the Death Criterion" Sensors 20, no. 21: 5981. https://doi.org/10.3390/s20215981