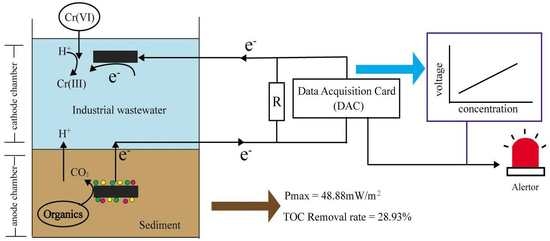
A Novel Early Warning System Based on a Sediment Microbial Fuel Cell for In Situ and Real Time Hexavalent Chromium Detection in Industrial Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Biosensor Setup and Operation
2.3. Analysis and Calculations
2.4. DNA Sequencing and Analysis
3. Results and Discussion
3.1. System Condition Optimization
3.2. Voltage Generated by the SMFC
3.3. Evaluation of the Biosensor Performance
3.3.1. Calibration of the Biosensor
3.3.2. Application of the Biosensor in Actual Wastewater Treatment
3.4. Scanning Electron Microscopy and Cyclic Voltammetry Analysis
3.5. Microbial Community Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hui, K.S.; Chao, C.Y.; Kot, S.C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Megharaj, M.; Avudainayagam, S.; Naidu, R. Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr. Microbiol. 2003, 47, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Chen, J.; Huang, Q.; Chen, J.; Xu, Y.; Luo, J.; Wang, K.; Gao, W.; Zheng, Y. Bioremediation of hexavalent chromium contaminated soil by a bioleaching system with weak magnetic fields. Int. Biodeterior. Biodegrad. 2016. [Google Scholar] [CrossRef]
- Ramirez-Diaz, M.I.; Diaz-Perez, C.; Vargas, E.; Riveros-Rosas, H.; Campos-Garcia, J.; Cervantes, C. Mechanisms of bacterial resistance to chromium compounds. Biometals 2008, 21, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, J.P. Biosorption of hexavalent chromium onto raw and chemically modified Sargassum sp. Bioresour. Technol. 2008, 99, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.-H.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Yari, A.; Bagheri, H. Determination of Cr (VI) with Selective Sensing of Cr (VI) Anions by a PVC-Membrane Electrode Based on Quinaldine Red. J. Chin. Chem. Soc. 2009, 56, 289–295. [Google Scholar] [CrossRef]
- Abdelfattah, I.; Ismail, A.A.; Sayed, F.A.; Almedolab, A.; Aboelghait, K.M. Biosorption of heavy metals ions in real industrial wastewater using peanut husk as efficient and cost effective adsorbent. Environ. Nanotechnol. Monit. Manag. 2016, 6, 176–183. [Google Scholar] [CrossRef]
- Gurung, A.; Oh, S.E.; Kim, K.D.; Shin, B.S. Semi-continuous detection of toxic hexavalent chromium using a sulfur-oxidizing bacteria biosensor. J. Environ. Manag. 2012, 106, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Han, H.; Liu, P.; Xiong, J.; Tian, F.; Li, X. Microbial Fuels Cell-Based Biosensor for Toxicity Detection: A Review. Sensors 2017, 17, 2230. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Tang, M.; Chen, X.; Qi, L.; Dong, S. Co-immobilized microbial biosensor for BOD estimation based on sol–gel derived composite material. Biosens. Bioelectron. 2003, 18, 1023–1029. [Google Scholar] [CrossRef]
- Jin, X.; Li, X.; Zhao, N.; Angelidaki, I.; Zhang, Y. Bio-electrolytic sensor for rapid monitoring of volatile fatty acids in anaerobic digestion process. Water Res. 2017, 111, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Bohrn, U.; Mucha, A.; Werner, C.F.; Trattner, B.; Bäcker, M.; Krumbe, C.; Schienle, M.; Stütz, E.; Schmitt-Landsiedel, D.; Fleischer, M.; et al. A critical comparison of cell-based sensor systems for the detection of Cr(VI) in aquatic environment. Sens. Actuators B: Chem. 2013, 182, 58–65. [Google Scholar] [CrossRef]
- Michel, C.; Ouerd, A.; Battaglia-Brunet, F.; Guigues, N.; Grasa, J.P.; Bruschi, M.; Ignatiadis, I. Cr(VI) quantification using an amperometric enzyme-based sensor: interference and physical and chemical factors controlling the biosensor response in ground waters. Biosens. Bioelectron. 2006, 22, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Singh, M. Biosensors for heavy metals. Biometals 2005, 18, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, W.; Mulchandani, A. Microbial biosensors. Anal. Chim. Acta 2006, 568, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Miran, W.; Nawaz, M.; Jang, J.; Lee, D.S. Sustainable electricity generation by biodegradation of low-cost lemon peel biomass in a dual chamber microbial fuel cell. Int. Biodeterior. Biodegrad. 2016, 106, 75–79. [Google Scholar] [CrossRef]
- Chen, Z.; Niu, Y.; Zhao, S.; Khan, A.; Ling, Z.; Chen, Y.; Liu, P.; Li, X. A novel biosensor for p-nitrophenol based on an aerobic anode microbial fuel cell. Biosens. Bioelectron. 2016, 85, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sik Hyun, M.; Gadd, G.M.; Joo Kim, H. A novel biomonitoring system using microbial fuel cells. J. Environ. Monit. 2007, 9, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Raut, N.; O’Connor, G.; Pasini, P.; Daunert, S. Engineered cells as biosensing systems in biomedical analysis. Anal. Bioanal. Chem. 2012, 402, 3147–3159. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Tsai, T.H.; Liu, M.H.; Kuo, J.L.; Chang, Y.C.; Chung, Y.C. A Green Microbial Fuel Cell-Based Biosensor for In Situ Chromium (VI) Measurement in Electroplating Wastewater. Sensors 2017, 17, 2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Cheng, C.Y.; Liu, M.H.; Chen, T.Y.; Hsieh, M.C.; Chung, Y.C. Utility of Ochrobactrum anthropi YC152 in a Microbial Fuel Cell as an Early Warning Device for Hexavalent Chromium Determination. Sensors 2016, 16, 1272. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Xu, M.; Liu, J.; Guo, J.; Yang, Y. Sediment microbial fuel cell prefers to degrade organic chemicals with higher polarity. Bioresour. Technol. 2015, 190, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Sherafatmand, M.; Ng, H.Y. Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresour. Technol. 2015, 195, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Song, N.; Cai, H.; Tay, J.H.; Jiang, H. Enhanced degradation of phenanthrene and pyrene in freshwater sediments by combined employment of sediment microbial fuel cell and amorphous ferric hydroxide. J. Hazard. Mater. 2012, 199–200, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Tender, L.M.; Reimers, C.E.; Stecher, H.A.; Holmes, D.E.; Bond, D.R.; Lowy, D.A.; Pilobello, K.; Fertig, S.J.; Lovley, D.R. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 2002, 20, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Donovan, C.; Dewan, A.; Peng, H.; Heo, D.; Beyenal, H. Power management system for a 2.5 W remote sensor powered by a sediment microbial fuel cell. J. Power Sources 2011, 196, 1171–1177. [Google Scholar] [CrossRef]
- Zhang, F.; Tian, L.; He, Z. Powering a wireless temperature sensor using sediment microbial fuel cells with vertical arrangement of electrodes. J. Power Sources 2011, 196, 9568–9573. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Electricity Production by Geobacter sulfurreducens Attached to Electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr. Opin. Biotechnol. 2006, 17, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Roman, O.B.; Angelidaki, I. Importance of temperature and anodic medium composition on microbial fuel cell (MFC) performance. Biotechnol. Lett. 2008, 30, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Chang, I.S.; Choi, Y.S.; Chung, T.H. Experimental evaluation of influential factors for electricity harvesting from sediment using microbial fuel cell. Bioresour. Technol. 2009, 100, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Pettine, M.; Capri, S. Removal of humic matter interference in the determination of Cr(VI) in soil extracts by the diphenylcarbazide method. Anal. Chim. Acta 2005, 540, 239–246. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, G.; Ghangrekar, M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Hotta, Y.; Watanabe, K. Methanogenesis versus electrogenesis: morphological and phylogenetic comparisons of microbial communities. Biosci. Biotechnol. Biochem. 2008, 72, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Rabaey, K.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Boon, N.; Verstraete, W. Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng. Life Sci. 2006, 6, 285–292. [Google Scholar] [CrossRef]
- Chung, H.; Ju, W.J.; Jho, E.H.; Nam, K. Applicability of a submersible microbial fuel cell for Cr(VI) detection in water. Environ. Monit. Assess. 2016, 188, 613. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, H.; Logan, B.E. Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ. Sci. Technol. 2006, 40, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, Z.; Lin, X.; Xia, C.; Sun, G.; Lian, Y.; Xu, M. Enhancing the bioremediation by harvesting electricity from the heavily contaminated sediments. Bioresour. Technol. 2015, 179, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.-M.; Tang, L.; Shen, G.-L.; Huang, G.-H.; Niu, C.-G. Determination of trace chromium(VI) by an inhibition-based enzyme biosensor incorporating an electropolymerized aniline membrane and ferrocene as electron transfer mediator. Int. J. Environ. Anal. Chem. 2004, 84, 761–774. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Kalathil, S.; Pant, D. Nanotechnology to rescue bacterial bidirectional extracellular electron transfer in bioelectrochemical systems. RSC Adv. 2016, 6, 30582–30597. [Google Scholar] [CrossRef]
- Clauwaert, P.; Aelterman, P.; Pham, T.H.; De Schamphelaire, L.; Carballa, M.; Rabaey, K.; Verstraete, W. Minimizing losses in bio-electrochemical systems: the road to applications. Appl. Microbiol. Biotechnol. 2008, 79, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Lu, D.; Wang, M.Y.; Chen, W.; Zhou, G.; Zhang, Y. Effect of chromium (VI) on the multiple nitrogen removal pathways and microbial community of aerobic granular sludge. Environ. Technol. 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, M.; Zhou, M.; Hu, Y. Characterization of a novel strain phylogenetically related to Kocuria rhizophila and its chemical modification to improve performance of microbial fuel cells. Biosens. Bioelectron. 2015, 69, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Seon, J.; Park, Y.; Cho, S.; Lee, T. Electricity generation and microbial community in a submerged-exchangeable microbial fuel cell system for low-strength domestic wastewater treatment. Bioresour. Technol. 2012, 117, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, S.; Jiao, K.; Hou, J.; Xie, H.; Xu, H. Bioremediation of soils co-contaminated with heavy metals and 2,4,5-trichlorophenol by fruiting body of Clitocybe maxima. J. Hazard. Mater. 2015, 294, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, D.; Ren, Z.J. Microbial community structure accompanied with electricity production in a constructed wetland plant microbial fuel cell. Bioresour. Technol. 2015, 195, 115–121. [Google Scholar] [CrossRef] [PubMed]








| Synthesized Wastewater | Real Wastewater | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Standard Cr(VI) Concentration (mg/L) | |||||||||
| 0.25 | 0.35 | 0.45 | 0.55 | 0.65 | 0.70 | a | b | c | |
| Colorimetric method | 0.24 ± 0.78 | 0.35 ± 1.02 | 0.46 ± 2.04 | 0.55 ± 0.68 | 0.64 ± 1.33 | 0.71 ± 2.34 | 0.46 ± 3.54 | 5.26 ± 4.32 | 24.63 ± 1.62 |
| SMFC-Biosensor | 0.25 ± 1.24 | 0.34 ± 2.23 | 0.47 ± 1.98 | 0.54 ± 0.86 | 0.67 ± 2.01 | 0.69 ± 1.08 | 0.48 ± 2.42 | 4.89 ± 2.07 | 26.18 ± 3.42 |
| D-value (C) | 0.01 | - | 0.01 | - | 0.01 | 0.01 | - | - | - |
| D-value (S) | 0.01 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | - | - | - |
| Deviation (%) | 4.17 | 2.86 | 2.17 | 1.82 | 4.69 | 2.82 | 4.35 | 7.03 | 6.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Liu, P.; Niu, Y.; Chen, Z.; Khan, A.; Zhang, P.; Li, X. A Novel Early Warning System Based on a Sediment Microbial Fuel Cell for In Situ and Real Time Hexavalent Chromium Detection in Industrial Wastewater. Sensors 2018, 18, 642. https://doi.org/10.3390/s18020642
Zhao S, Liu P, Niu Y, Chen Z, Khan A, Zhang P, Li X. A Novel Early Warning System Based on a Sediment Microbial Fuel Cell for In Situ and Real Time Hexavalent Chromium Detection in Industrial Wastewater. Sensors. 2018; 18(2):642. https://doi.org/10.3390/s18020642
Chicago/Turabian StyleZhao, Shuai, Pu Liu, Yongyan Niu, Zhengjun Chen, Aman Khan, Pengyun Zhang, and Xiangkai Li. 2018. "A Novel Early Warning System Based on a Sediment Microbial Fuel Cell for In Situ and Real Time Hexavalent Chromium Detection in Industrial Wastewater" Sensors 18, no. 2: 642. https://doi.org/10.3390/s18020642