A Microfluidic Approach for Inducing Cell Rotation by Means of Hydrodynamic Forces
Abstract
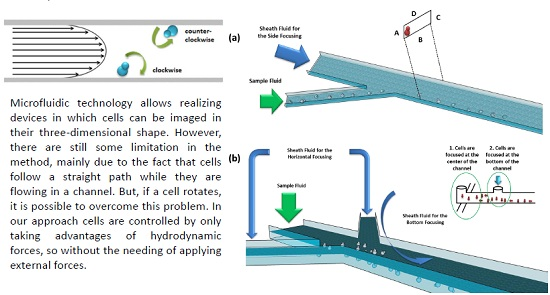
:1. Introduction
1.1. Electrical Manipulation
1.2. Optical Manipulation
1.3. Acoustophoresis
1.4. Magnetofluidic Trapping
1.5. Hydrodynamic Manipulation
2. Theoretical Background
- -
- the particle coordinate system with its origin being at the particle mass center and its axes being the principal axes x, y, and z.
- -
- the deformation rate tensor , the spin rate tensor, and defined as follows:
- -
- and are, respectively, the minor axis and the linear velocity of the ellipsoidal particle.
- -
- , the ratio between the major and minor axes of the ellipsoidal particle, and from this definition , , and :
- -
- The angular velocity components are:with , , the Euler’s angles [48].
3. Materials and Methods
3.1. Numerical Simulation Analysis
3.2. Design and Fabrication
3.3. Experimental Setup
4. Results
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huh, D.; Gu, W.; Kamotani, Y.; Grotberg, J.B.; Takayama, S. Microfluidics for flow cytometric analysis of cells and particles. Physiol. Meas. 2005. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, C.; Groisman, A. High-throughput and high-Resolution flow cytometry in molded microfluidic devices. Anal. Chem. 2006, 78, 5653–5663. [Google Scholar] [CrossRef] [PubMed]
- Watkins, N.; Venkatesan, B.; Tone, M.; Rodriguez, W.; Bashir, R. A robust electrical micro-cytometer with 3-dimensional hydrofocusing. Lab Chip 2009, 9, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Nawaz, A.A.; Lin, S.C.; Lapsley, M.I.; Zhao, Y.; McCoy, J.P.; El-Deiry, W.S.; Huang, T.J. An integrated, multiparametric flow cytometry chip using “microfluidic drifting” based three-dimensional hydrodynamic focusing. Biomicrofluidics 2012, 6, 24113–241139. [Google Scholar] [CrossRef] [PubMed]
- Schonbrun, E.; Malka, R.; Di Caprio, G.; Schaak, D.; Higgins, J.M. Quantitative absorption cytometry for measuring red blood cell hemoglobin mass and volume. Cytom. Part A 2009, 85, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Han, S.I.; Joo, Y.D.; Han, K.H. An electrorotation technique for measuring the dielectric properties of cells with simultaneous use of negative quadrupolar dielectrophoresis and electrorotation. Analyst 2013, 138, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Chau, L.H.; Liang, W.; Cheung, F.W.K.; Liu, W.K.; Li, W.J.; Chen, S.C.; Lee, G.B. Self-rotation of cells in an irrotational AC E-field in an opto-electrokinetics chip. PLoS ONE 2013, 8, e51577. [Google Scholar] [CrossRef] [PubMed]
- Tabeling, P. Introduction to Microfluidics, 6th ed.; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Pethig, R. Dielectrophoresis: Using Inhomogeneous AC Electrical Fields to Separate and Manipulate Cells. Crit. Rev. Biotechnol. 1996, 6, 331–334. [Google Scholar] [CrossRef]
- Shafiee, H.; Caldwell, J.L.; Sano, M.B.; Davalos, R.V. Contactless dielectrophoresis: A new technique for cell manipulation. Biomed. Microdevices 2009, 11, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Flanagan, L.A.; Jeon, N.L.; Monuki, E.; Lee, A.P. Dielectrophoresis switching with vertical sidewall electrodes for microfluidic flow cytometry. Lab Chip 2007, 7, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Benhal, P.; Chase, J.G.; Gaynor, P.; Oback, B.; Wang, W. AC electric field induced dipole-based on-chip 3D cell rotation. Lab Chip 2014, 14, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A.; Dziedzic, J.M. Optical Levitation by Radiation Pressure. Appl. Phys. Lett. 1971, 19, 283. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M. Optical trapping and manipulation of viruses and bacteria. Science 1987, 235, 151. [Google Scholar] [CrossRef]
- Neuman, K.C.; Block, S.M. Optical Trapping. Rev. Sci. Instrum. 2004, 75, 2787–2890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K.K. Optical Tweezers for single cells. J. R. Soc. Interface 2008, 5, 671. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Yoon, S.Y.; Sung, H.J.; Kim, S.S. Cross-Type Optical Particle Separation in a Microchannel. Anal. Chem. 2008, 80, 2628–2630. [Google Scholar] [CrossRef] [PubMed]
- Guck, J.; Ananthakrishnan, R.; Mahmood, H.; Moon, T.J.; Cunningham, C.C.; Kas, J. Stretching biological cells with light. J. Phys. Condens. Matter 2002, 14, 4843–4856. [Google Scholar] [CrossRef]
- Lincoln, B.; Schinkinger, S.; Travis, K.; Wottawah, F.; Ebert, S.; Sauer, F.; Guck, J. Reconfigurable microfluidic integration of a dual-beam laser trap with biomedical applications. Biomed. Microdevices 2007, 9, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.; Albert, S.; Haug, M.; Whyte, G. Optofluidic rotation of living cells for single-cell tomography. J. Biophotonics 2015, 8, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, R.; Ahlawat, S.; Verma, R.S.; Gupta, P.K. Optical orientation and rotation of trapped red blood cells with Laguerre-Gaussian mode. Opt. Express 2011, 19, 7680–7688. [Google Scholar] [CrossRef] [PubMed]
- Kreysing, M.K.; Kiessling, T.; Fritsch, A.; Dietrich, C.; Guck, J.R.; Käs, J.A. The optical cell rotator. Opt. Express 2008, 16, 16984–16992. [Google Scholar] [CrossRef] [PubMed]
- Bruus, H. Acoustofluidics 1: Governing equations in microfluidics. Lab Chip 2011, 11, 3742–3751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.; Feke, D. Methodology for fractionating suspended particles using ultrasonic standing wave and divided flow fields. Sep. Technol. 1995, 5, 251–258. [Google Scholar] [CrossRef]
- Yasuda, K.; Umemura, S.; Takeda, K. Concentration and fractionation of small particles in liquid by ultrasound. Jpn. J. Appl. Phys. 1995, 34, 2715–2720. [Google Scholar] [CrossRef]
- Hawkes, J.J.; Coakley, W.T. Force field particle filter, combining ultrasound standing waves and laminar flow. Sens. Actuators B 2001, 75, 213–222. [Google Scholar] [CrossRef]
- Petersson, F.; Nilsson, A.; Holm, C.; Jönsson, H.; Laurell, T. Separation of lipids from blood utilizing ultrasonic standing waves in microfluidic channels. Analyst 2004, 129, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Petersson, F.; Nilsson, A.; Holm, C.; Jönsson, H.; Laurell, T. Continuous separation of lipid particles from erythrocytes by means of laminar flow and acoustic standing wave forces. Lab Chip 2005, 5, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Barnkob, R.; Laub Busk, L.; Tidemand-Lichtenberg, P.; Bruus, H.; Berg-Sørensen, K. Optical stretching on chip with acoustophoretic prefocusing. Proc. SPIE 2012. [Google Scholar] [CrossRef]
- Li, S.; Ren, L.; Huang, P.H.; Yao, X.; Cuento, R.A.; McCoy, J.P.; Cameron, G.E.; Levine, S.J.; Huang, T.J. Acoustofluidic Transfer of Inflammatory Cells from Human Sputum Samples. Anal. Chem. 2016, 88, 5655–5661. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Ozcelik, A.; Bojanala, N.; Nama, N.; Upadhyay, A.; Chen, Y.; Hanna-Rose, W.; Huang, T.J. Rotational manipulation of single cells and organisms using acoustic waves. Nat. Commun. 2016, 7, 11085. [Google Scholar] [CrossRef] [PubMed]
- Pamme, N. Magnetism and microfluidics. Lab Chip 2006, 6, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Megias-Alguacil, D. Surface rotation of liquid droplets under a simple shear flow: Experimental observation in 3D. Soft Mater. 2013, 11, 1–5. [Google Scholar] [CrossRef]
- Lednev, V.V. Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics 1991, 12, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Hejazian, M.; Nguyen, N.T. Magnetofluidic concentration and separation of non-magnetic particles using two magnet arrays. Biomicrofluidics 2016, 10, 044103. [Google Scholar] [CrossRef] [PubMed]
- Tanyeri, M.; Johnson-Chavarria, E.M.; Schroeder, C.M. Hydrodynamic trap for single particles and cells. Appl. Phys. Lett. 2010, 96, 224101–224103. [Google Scholar] [CrossRef] [PubMed]
- Sipos, O.; Nagy, K.; Di Leonardo, R.; Galajda, P. Hydrodynamic Trapping of Swimming Bacteria by Convex Walls. Phys. Rev. Lett. 2015, 114, 258104–258109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelby, J.P.; Chiu, D.T. Controlled rotation of biological micro- and nano-particles in microvortices. Lab Chip 2004, 4, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.W.; Shelby, J.P.; Kuo, J.S.; Chiu, D.T. Dynamic formation of ring-shaped patterns of colloidal particles in microfluidic systems. Appl. Phys. Lett. 2003, 83, 1145. [Google Scholar] [CrossRef]
- Hagiwara, M.; Kawahara, T.; Arai, F. Local streamline generation by mechanical oscillation in a microfluidic chip for noncontact cell manipulations. Appl. Phys. Lett. 2012, 101, 074102. [Google Scholar] [CrossRef]
- Lutz, B.R.; Chen, J.; Schwartz, D.T. Characterizing Homogeneous Chemistry Using Well-Mixed Microeddies. Anal. Chem. 2006, 78, 5429–5435. [Google Scholar] [CrossRef] [PubMed]
- Bruus, H. Theoretical Microfluidics (Oxford Master Series in Physics), 1st ed.; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.T.; Warkianic, M.E.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D. Inertial Microfluidics. Lab Chip 2009, 9, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Wereley, S.T. Fundamental and Applications of Microfluidics, 2nd ed.; Artech House: Norwood, MA, USA, 2002. [Google Scholar]
- Feng, Y.; Kleinstreuer, C. Analysis of non-spherical particle transport in complex internal shear flows. Phys. Fluids 2013, 25, 091904. [Google Scholar] [CrossRef]
- Jefferey, J.B. The motion of ellipsoidal particles immersed in a viscous fluid. Proc. Royal Soc. A 1922, 102, 161–179. [Google Scholar] [CrossRef]
- Goldstein, H.; Poole, C.P.; Safko, J. Classical Mechanics; Addison-Wesley Publishing Company: Boston, MA, USA, 2001. [Google Scholar]
- Alberts, B.; Bray, D.; Hopkin, K.; Johnson, A.D.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Essential Cell Biology, 6th ed.; Garland Science: New York, NY, USA, 2013. [Google Scholar]










© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torino, S.; Iodice, M.; Rendina, I.; Coppola, G.; Schonbrun, E. A Microfluidic Approach for Inducing Cell Rotation by Means of Hydrodynamic Forces. Sensors 2016, 16, 1326. https://doi.org/10.3390/s16081326
Torino S, Iodice M, Rendina I, Coppola G, Schonbrun E. A Microfluidic Approach for Inducing Cell Rotation by Means of Hydrodynamic Forces. Sensors. 2016; 16(8):1326. https://doi.org/10.3390/s16081326
Chicago/Turabian StyleTorino, Stefania, Mario Iodice, Ivo Rendina, Giuseppe Coppola, and Ethan Schonbrun. 2016. "A Microfluidic Approach for Inducing Cell Rotation by Means of Hydrodynamic Forces" Sensors 16, no. 8: 1326. https://doi.org/10.3390/s16081326