Improved Adhesion Performance of Soy Protein-Based Adhesives with a Larch Tannin-Based Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TR
2.3. Preparation of SPAs
2.4. Characterization of SPAs
2.4.1. Rheology Properties
2.4.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Moisture Uptake Measurement
2.4.5. Residual Rate Test
2.5. Preparation of the Plywood Samples
2.6. Shear Strength Measurement of Plywood
2.7. Statistic Analysis
3. Results
3.1. Characteristic of Tannin and TR Samples
3.1.1. Physical Properties of TR Samples
3.1.2. FTIR Spectroscopic Analysis of Tannin and TR
3.1.3. Thermal Behavior of Tannin and TR
3.2. Viscosity of the Adhesive Samples
3.3. FTIR Spectroscopic Analysis of the Adhesvie Samples
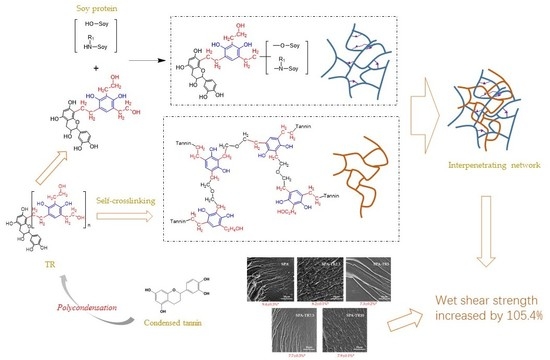
3.4. SEM Analysis of the Adhesive Samples
3.5. Shear Strength of Plywood
4. Conclusions
- (1)
- The addition of 5 wt % TR in SPA increased the residual rate of the resultant adhesive by 38.4% and the wet shear strength of the resultant plywood by 105.4%;
- (2)
- The water resistance and the crosslinking density of the adhesive were improved because of the following mechanisms: (a) a crosslinking structure formed through the reaction between the TR and the soy protein molecules; (b) An interpenetrated network formed through the crosslinking protein molecules and the self-crosslinking TR molecules; (c) NaOH in TR made the soy protein more soluble and more ionic amino acids, which increased the reactive sites of soy protein with crosslinkers, thus increasing the crosslinking density;
- (3)
- The viscosity improved, which was beneficial for the adhesive distribution during the hot press process. This formed a stronger interlock with the wood surface, which resulted in a better water resistance and bonding strength obtained in the resultant adhesive; and
- (4)
- The addition of 10 wt % TR in SPA decreased the bonding strength of the resultant adhesive because the quantity of unfolded protein molecular chains that caused the resultant adhesive to have a very high viscosity. This caused the adhesive to adhere the wood surface non-uniformly, which did not give the resultant plywood effective bonding strength.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koshy, R.; Mary, S.; Thomas, S.; Pothan, L. Environment friendly green composites based on soy protein isolate—A review. Food Hydrocoll. 2015, 50, 174–192. [Google Scholar] [CrossRef]
- He, Z. Bio-Based Wood Adhesives—Preparation, Characterization, and Testing; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 5–9. [Google Scholar]
- Ren, X.; Soucek, M. Soy-based coatings and adhesives. ACS Symp. 2014, 1178, 207–254. [Google Scholar]
- Nishinari, K.; Fang, Y.; Guo, S.; Phillips, G. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Qi, G.; Li, N.; Wang, D.; Sun, X. Physicochemical properties of soy protein adhesives modified by 2-octen-1-ylsuccinic anhydride. Ind. Crops Prod. 2013, 46, 165–172. [Google Scholar] [CrossRef]
- Chen, N.; Lin, Q.; Rao, J.; Zeng, Q. Water resistances and bonding strengths of soy-based adhesives containing different carbohydrates. Ind. Crops Prod. 2013, 50, 44–49. [Google Scholar] [CrossRef]
- Mo, X.; Sun, X. Soy proteins as plywood adhesives: Formulation and characterization. J. Adhes. Sci. Technol. 2013, 27, 2014–2026. [Google Scholar] [CrossRef]
- Qi, G.; Sun, X. Soy protein adhesive blends with synthetic latex on wood veneer. J. Am. Oil Chem. Soc. 2011, 88, 271–281. [Google Scholar] [CrossRef]
- Gui, C.; Wang, G.; Wu, D.; Zhu, J.; Liu, X. Synthesis of a bio-based polyamidoamine-epichlorohydrin resin and its application for soy-based adhesives. Int. J. Adhes. Adhes. 2013, 44, 237–242. [Google Scholar] [CrossRef]
- Luo, J.; Li, C.; Li, X.; Luo, J.; Gao, Q.; Li, J. A new soybean meal-based bioadhesive enhanced with 5,5-dimethyl hydantoin polyepoxide for the improved water resistance of plywood. RSC Adv. 2015, 5, 62957–62965. [Google Scholar] [CrossRef]
- Lei, H.; Wu, Z.; Cao, M.; Du, G. Study on the soy protein-based wood adhesive modified by hydroxymethyl phenol. Polymers 2016, 8, 256. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Yuan, C.; Zhang, W.; Li, J.; Gao, Q. An eco-friendly wood adhesive from soy protein and lignin: Performance properties. RSC Adv. 2015, 5, 100849–100855. [Google Scholar] [CrossRef]
- Ping, L.; Pizzi, A.; Guo, Z.; Brosse, N. Condensed tannins from grape pomace:characterization by FTIR and MALDI TOF and production of environment friendly wood adhesive. Ind. Crops Prod. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- Tondi, G.; Schnabel, T.; Wieland, S.; Petutschnigg, A. Surface properties of tannin treated wood during natural and artificial weathering. Int. Wood Prod. J. 2013, 4, 150–157. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, H.; Gao, Q.; Li, J.; Pizzi, A.; Delmotte, L. Performances of larch (larix gmelini) tannin modified urea–formaldehyde (TUF) resin and plywood bonded by TUF resin. J. Appl. Polym. Sci. 2014, 131, 547–557. [Google Scholar] [CrossRef]
- García, D.; Glasser, W.; Pizzi, A.; Lacoste, C.; Laborie, M. Polyphenolic resins prepared with maritime pine bark tannin and bulky-aldehydes. Ind. Crops Prod. 2014, 62, 84–93. [Google Scholar] [CrossRef]
- Saad, H.; Khoukh, A.; Ayed, N.; Charrier, B.; Bouhtoury, F. Characterization of Tunisian Aleppo pine tannins for a potential use in wood adhesive formulation. Ind. Crops Prod. 2014, 61, 517–525. [Google Scholar] [CrossRef]
- Zhou, X.; Pizzi, A.; Sauget, A.; Nicollin, A.; Li, X.; Celzard, A.; Rode, K.; Pasch, H. Lightweight tannin foam/composites sandwich panels and the coldset tannin adhesive to assemble them. Ind. Crops Prod. 2013, 43, 255–260. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Gao, Q.; Li, J. Effects of heat treatment on wet shear strength of plywood bonded with soybean meal-based adhesive. Ind. Crops Prod. 2015, 63, 281–286. [Google Scholar] [CrossRef]
- Test Methods of Evaluating the Properties of Wood—Based Panels and Surface Decorated Wood-Based Panels. Available online: http://www.anystandards.com/gbt/8/20140331/43590.html (accessed on 12 November 2013).
- Ricci, A.; Lagel, M.; Parpinello, G.; Pizzi, A.; Kilmartin, P.; Versari, A. Spectroscopy analysis of phenolic and sugar patterns in a food grade chestnut tannin. Food Chem. 2016, 203, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Tondi, G.; Petutschnigg, A. Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts. Ind. Crops Prod. 2015, 65, 422–428. [Google Scholar] [CrossRef]
- Pizzi, A.; Pasch, H.; Celzard, A.; Szczurek, A. Oligomer Distribution at the gel point of tannin-resorcinol-formaldehyde cold-set wood adhesives. J. Adhes. Sci. Technol. 2012, 26, 79–88. [Google Scholar]
- Chupin, L.; Charrier, B.; Pizzi, A.; Perdomo, A.; Charrier, F. Study of thermal durability properties of tannin-lignosulfonate adhesives. J. Therm. Anal. Calorim. 2015, 119, 1577–1585. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. A novel water-based process produces eco-friendly bio-adhesive made from green cross-linked soybean soluble polysaccharide and soy protein. Carbohydr. Polym. 2017, 169, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupe, A.; Poliszuk, A.; Eisenberg, P.; Escobar, M. Rheological behavior and bonding performance of an alkaline soy protein suspension. Int. J. Adhes. Adhes. 2015, 62, 1–6. [Google Scholar] [CrossRef]
- Chen, X.; Ru, Y.; Chen, F.; Wang, X.; Zhao, X.; Ao, Q. FTIR spectroscopic characterization of soy proteins obtained through AOT reverse micelles. Food Hydrocoll. 2013, 31, 435–437. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Li, X.; Yi, Z.; Gao, Q.; Li, J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crops Prod. 2015, 74, 613–618. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Li, J.; Gao, Q. Investigating the use of peanut meal: A potential new resource for wood adhesives. RSC Adv. 2015, 5, 80136–80141. [Google Scholar] [CrossRef]
- Chen, N.; Zeng, Q.; Lin, Q.; Rao, J. Development of defatted soy flour based bio-adhesives using Viscozyme. Ind. Crops Prod. 2015, 76, 198–203. [Google Scholar] [CrossRef]
- Neto, W.; Silverio, H.; Dantas, N.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue—Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Wang, D.; Sun, X.; Yang, G. Improved water resistance of soy protein adhesive at isoelectric point. Trans. ASABE 2009, 52, 173–177. [Google Scholar] [CrossRef]






| Resin | Solid Content | Viscosity | pH |
|---|---|---|---|
| TR | 57.1 ± 0.3% | 970 ± 9 mPa·s | 10.0 ± 0.2 |
| Adhesive Samples | SPA | SPA-TR2.5 | SPA-TR5 | SPA-TR7.5 | SPA-TR10 |
|---|---|---|---|---|---|
| Viscosity (mPa·s) | 111,900 ± 1357 | 45,800 ± 689 | 71,010 ± 476 | 94,790 ± 413 | 105,512 ± 978 |
| pH value | 7.2 ± 0.2 | 7.5 ± 0.1 | 7.9 ± 0.2 | 8.2 ± 0.1 | 8.6 ± 0.1 |
| Adhesive Samples | SPA | SPA-TR2.5 | SPA-TR5 | SPA-TR7.5 | SPA-TR10 |
|---|---|---|---|---|---|
| Dry shear strength (MPa) | 1.24 ± 0.10 | 1.83 ± 0.15 | 2.27 ± 0.13 | 2.02 ± 0.11 | 1.74 ± 0.12 |
| Wood failure | 10% | 40% | 70% | 50% | 30% |
| Wet shear strength (MPa) | 0.55 ± 0.02 | 0.87 ± 0.03 | 1.13 ± 0.08 | 0.98 ± 0.04 | 0.79 ± 0.05 |
| Wood failure | 0 | 30% | 50% | 30% | 10% |
| Residual rate | 61.8 ± 0.6% | 81.2 ± 1.0% | 83.7 ± 0.4% | 79.5 ± 0.4% | 72.3 ± 0.4% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Luo, J.; Shi, R.; Zhang, J.; Gao, Q.; Li, J. Improved Adhesion Performance of Soy Protein-Based Adhesives with a Larch Tannin-Based Resin. Polymers 2017, 9, 408. https://doi.org/10.3390/polym9090408
Chen M, Luo J, Shi R, Zhang J, Gao Q, Li J. Improved Adhesion Performance of Soy Protein-Based Adhesives with a Larch Tannin-Based Resin. Polymers. 2017; 9(9):408. https://doi.org/10.3390/polym9090408
Chicago/Turabian StyleChen, Mingsong, Jing Luo, Ruiqing Shi, Jizhi Zhang, Qiang Gao, and Jianzhang Li. 2017. "Improved Adhesion Performance of Soy Protein-Based Adhesives with a Larch Tannin-Based Resin" Polymers 9, no. 9: 408. https://doi.org/10.3390/polym9090408