Self-Assembled Behavior of Ultralightweight Aerogel from a Mixture of CNC/CNF from Oil Palm Empty Fruit Bunches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
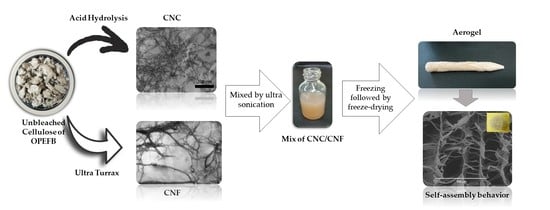
2.2.1. Fabrication of Aerogel-Based CNC/CNF Mixture
2.2.2. Characterization
3. Results and Discussion
3.1. Chemical Structure
3.2. Morphology
3.3. Specific Surface Area
3.4. Crystallinity Degree
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Amphiphilic superabsorbent cellulose nanofibril aerogels. J. Mater. Chem. A 2014, 2, 6337–6342. [Google Scholar] [CrossRef] [Green Version]
- Rafieian, F.; Hosseini, M.; Jonoobi, M.; Yu, Q. Development of hydrophobic nanocellulose-based aerogel via chemical vapor deposition for oil separation for water treatment. Cellulose 2018, 25, 4695–4710. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Li, C.; Liang, H.W.; Chen, J.F.; Yu, S.H. Ultralight, flexible, and fire-resistant carbon nanofiber aerogels from bacterial cellulose. Angew. Chem. Int. Ed. 2013, 52, 2925–2929. [Google Scholar] [CrossRef]
- Wu, W.; Tassi, N.G.; ZHU, H.; Fang, Z.; Hu, L. Nanocellulose-based Translucent Diffuser for Optoelectronic Device Applications with Dramatic Improvement of Light Coupling. ACS Appl. Mater. Interfaces 2015, 7, 26860–26864. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhang, Z.; Song, F.; Zhang, W.; Li, H.; Gu, J.; Liu, Q.; Zhang, D. Ultralight, flexible carbon hybrid aerogels from bacterial cellulose for strong microwave absorption. Carbon 2020, 162, 283–291. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, X.; Li, H.; Zhou, D.; Wu, Q. Bio-Composites Consisting of Cellulose Nanofibers and Na+ Montmorillonite Clay: Morphology and Performance Property. Polymers 2020, 12, 1448. [Google Scholar] [CrossRef]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.; Serimaa, R.; Kuga, S.; Hirvonen, J.T.; Laaksonen, T. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef]
- Leitch, M.E.; Li, C.; Ikkala, O.; Mauter, M.S.; Lowry, G.V. Bacterial Nanocellulose Aerogel Membranes: Novel High-Porosity Materials for Membrane Distillation. Environ. Sci. Technol. Lett. 2016, 3, 85–91. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Nakagaito, A.; Kondo, H.; Takagi, H. Cellulose nanofibers aerogel production and applications. J. Reinf. Plast. Compos. 2013, 21, 699–709. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, Z.; Wang, H. The effect of freezing speed and hydrogel concentration on the microstructure and compressive performance of bamboo-based cellulose aerogel. J. Wood Sci. 2015, 61, 595–601. [Google Scholar] [CrossRef]
- Barrios, E.; Fox, D.; Sip, Y.Y.L.; Catarata, R.; Calderon, J.E.; Azim, N.; Afrin, S.; Zhang, Z.; Zhai, L. Nanomaterials in advanced, high-performance aerogel composites: A review. Polymers 2019, 11, 726. [Google Scholar] [CrossRef] [Green Version]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Khalil, H.A.; Davoudpour, Y.; Saurabh, C.; Hossain, S.; Adnan, A.S.; Dungani, R.; Paridah, M.; Sarker, Z.I.; Fazita, M.N.; Syakir, M.; et al. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Phanthond, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A. Nanocellulose: Extraction and application. Carbo Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Usmani, M.; Khan, I.; Gazal, U.; Haafiz, M.M.; Bhat, A. Interplay of Polymer Bionanocomposites and Significance of Ionic Liquids for Heavy Metal Removal; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 441–463. [Google Scholar]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, R.; Lindstrom, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Bras, J. Use of nanocellulose in printed electronics: A review. Nanoscale 2016, 8, 13131–13154. [Google Scholar] [CrossRef]
- Hasan, M.; Gopakumar, D.A.; Arumughan, V.; Pottathara, Y.B.; Sisanth, K.S.; Pasquini, D.; Bračič, M.; Seantier, M.; Nzihou, A.; Thomas, S.; et al. Robust superhydrophobic cellulose nanofiber aerogel for multifunctional environmental applications. Polymers 2019, 11, 495. [Google Scholar]
- Laitinen, O.; Suopajärvi, T.; Sirviö, J.A.; Liimatainen, H. Superabsorbent Aerogels from Cellulose Nanofibril Hydrogels. In Encyclopedia of Polymers and Composites; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 575–600. [Google Scholar]
- Zhou, L.; Zhai, S.; Chen, Y.; Xu, Z. Anisotropic Cellulose Nanofibers/Polyvinyl Alcohol/Graphene Aerogels Fabricated by Directional. Polymers 2019, 11, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, L.; Thielemans, W. Cellulose nanowhisker aerogels. Green Chem. 2010, 21, 1449–1463. [Google Scholar] [CrossRef]
- Shamskar, K.R.; Heidari, H.; Rashidi, A. Preparation and evaluation of nanocrystalline cellulose aerogels from raw cotton and cotton stalk. Ind. Crop. Prod. 2016, 93, 203–211. [Google Scholar] [CrossRef]
- Abraham, E.; Weber, D.E.; Sharon, S.; Lapidot, S.; Shoseyov, O. Multifunctional Cellulosic Scaffolds from Modified Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 2010–2015. [Google Scholar] [CrossRef]
- Buesch, C.; Smith, S.W.; Eschbach, P.; Conley, J.J.F.; Simonsen, J. The Microstructure of Cellulose Nanocrystal Aerogels as Revealed by Transmission Electron Microscope Tomography. Biomacromolecules 2016, 17, 2956–2962. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Jiang, H.; Song, Y.; Zhou, Z.; Zhao, H. Fabrication and characterization of nano-cellulose aerogels via supercritical CO2 drying technology. Mater. Lett. 2016, 183, 179–182. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Spray freeze-dried nanofibrillated cellulose aerogels with thermal superinsulating properties. Carbohydr. Polym. 2017, 157, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Munier, P.; Gordeyeva, K.; Bergström, L.; Fall, A.B. Directional Freezing of Nanocellulose Dispersions Aligns the Rod-Like Particles and Produces Low-Density and Robust Particle Networks. Biomacromolecules 2016, 17, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Nyström, G.; Marais, A.; Karabulut, E.; Wågberg, L.; Cui, Y.; Hamedi, M.M. Self-assembled three-dimensional and com-pressible interdigitated thin-film supercapacitors and batteries. Nat. Commun. 2015, 6, 7259. [Google Scholar] [CrossRef] [Green Version]
- Toivonen, M.S.; Kaskela, A.; Rojas, O.J.; Kauppinen, E.I.; Ikkala, O. Ambient-Dried Cellulose Nanofi bril Aerogel Mem-branes with High Tensile Strength and Their Use for Aerosol Collection and Templates for Transparent, Flexible Devices. Adv. Funct. Mater. 2015, 25, 6618–6626. [Google Scholar] [CrossRef] [Green Version]
- Zanini, M.; Lavoratti, A.; Lazzari, L.K.; Galiotto, D.; Baldasso, C.; Zattera, A.J. Obtaining hydrophobic aerogels of un-bleached cellulose nanofibers of the species eucalyptus sp. and pinus elliottii. J. Nanomater. 2018, 2018, 11. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, P.; Wang, M.; Zhao, H.; Yang, J.; Xu, F. Sustainable, Reusable, and Superhydrophobic Aerogels from Micro fibrillated Cellulose for Highly Effective Oil/Water Separation. ACS Sustain. Chem. Eng. 2016, 4, 6409–6416. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Zou, L.; Wang, F.; Wang, X.; Zhang, Y.; Yao, X. Nanocellulose-derived highly porous carbon aerogels for supercapacitors. Carbon 2016, 99, 203–211. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Li, C.; Liang, H.-W.; Zhang, Y.-N.; Wang, X.; Chen, J.-F.; Yu, S.-H. Carbon nanofiber aerogels for emergent cleanup of oil spillage and chemical leakage under harsh conditions. Sci. Rep. 2014, 3, 4079. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Wang, X.; Liu, S.; Yao, Y. Characterization of the nano-cellulose aerogel from mixing CNF and CNC with different ratio. Mater. Lett. 2018, 229, 103–106. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Super water absorbing and shape memory nanocellulose aerogels from TEMPO-oxidized cellulose nanofibrils via cyclic freezing-thawing. J. Mater. Chem. A 2014, 2, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Yu, L.; Tian, F.; Zhao, N.; Li, X.; Bian, F.; Wang, J. Cellulose nanofibrils aerogels generated from jute fibers. Carbohydr. Polym. 2014, 109, 35–43. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Wang, Y.; Yi, X.; Zeng, J.; Yu, H.; Liu, Y.; Li, J. Comparative Study of Aerogels Obtained from Differently Prepared Nanocellulose Fibers. ChemSusChem 2014, 7, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Gao, R.; Lu, Y.; Li, J.; Sun, Q. Fabrication and characterization of nanofibrillated cellulose and its aerogels from natural pine needles. Carbohydr. Polym. 2015, 119, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Xu, G.; Xu, Y.; Wang, F.; Shen, H. Ultralitght, hydrophobic, sustainable, cost-effective and floating kapok/microfibrillated cellulose aerogels as speedy and recyclable oil superadsorbents. J. Hazard. Mater. 2020, 406, 124758. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Huang, R.; Zhang, Y.; Jia, C.; Zhao, H.; Chen, W.; Xue, Y. Fabrication and Characterization of Cellulose Nanofibers Aerogels Prepared via Two Different Drying Techniques. Polymers 2020, 12, 2583. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://landportal.org/library/resources/issn-0126-2912-no-publikasipublication-number-032201911-katalogcatalog-1101001 (accessed on 8 August 2021).
- Chang, S.H. An overview of empty fruit bunch from oil palm as feedstock for bio-oil production. Biomass Bioenergy 2014, 62, 174–181. [Google Scholar] [CrossRef]
- Burhani, D.; Septevani, A.A. Isolation of Nanocellulose from Oil Palm Empty Fruit Bunches Using Strong Acid Hydrolysis. AIP Conf. Proc. 2018, 2024, 020005. [Google Scholar]
- Septevani, A.A.; Burhani, D.; Sudiyarmanto, S. Pengaruh Proses Pemutihan Multi Tahap Serat Selulosa Dari Limbah Tandan Kosong Kelapa Sawit. J. Kim. Kemasan 2018, 40, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Isolation, preparation, and characterization of nanofibers from oil palm empty-fruit-bunch (OPEFB). Cellulose 2010, 17, 977–985. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, A.; Johari, A.; Jusoh, M. Isolation, Characterization and Application of Nanocellulose from Oil Palm Empty Fruit Bunch as Nanocomposites. J. Nanomater 2014, 2014, 702538. [Google Scholar] [CrossRef] [Green Version]
- Septevani, A.A.; Rifathin, A.; Sari, A.A.; Sampora, Y.; Ariani, G.N.; Sudiyarmanto; Sondari, D. Oil palm empty fruit bunch-based nanocellulose as a super-adsorbent for water remediation. Carbohydr. Polym. 2020, 229, 115433. [Google Scholar] [CrossRef] [PubMed]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nat. Cell Biol. 1931, 127, 741. [Google Scholar] [CrossRef]
- Beck, S.; Bouchard, J.; Berry, R. Dispersibility in Water of Dried Nanocrystalline Cellulose. Biomacromolecules 2012, 13, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Osorio, D.A.; Seifried, B.; Moquin, P.; Grandfield, K.; Cranston, E.D. Morphology of cross-linked cellulose nanocrystal aerogels: Cryo-templating versus pressurized gas expansion processing. J. Mater. Sci. 2018, 53, 9842–9860. [Google Scholar] [CrossRef]
- Morán, J.I.; Álvarez, V.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2007, 15, 149–159. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef]
- Haafiz, M.M.; Hassan, A.; Zakaria, Z.; Inuwa, I. Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr. Polym. 2014, 103, 119–125. [Google Scholar] [CrossRef]
- Jonoobi, M.; Khazaeian, A.; Tahir, P.M.; Azry, S.S.; Oksman, K. Characteristics of cellulose nanofibers isolated from rub-berwood and empty fruit bunches of oil palm using chemo-mechanical process. Cellulose 2011, 18, 1085–1095. [Google Scholar] [CrossRef]
- Kaushik, A.; Singh, M. Isolation and characterization of cellulose nanofibrils from wheat straw using steam explosion coupled with high shear homogenization. Carbohydr. Res. 2011, 346, 76–85. [Google Scholar] [CrossRef]
- Chieng, B.W.; Lee, S.H.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Isolation and Characterization of Cellulose Nanocrystals from Oil Palm Mesocarp Fiber. Polymers 2017, 9, 355. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, S.; Goenaga, G.A.; Meng, X.; Zawodzinski, T.A.; Ragauskas, A.J. Chemically Cross-Linked Cellulose Nanocrystal Aerogels for Effective Removal of Cation Dye. Front. Chem. 2020, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; You, T.; Zhang, X.; Xu, F. Superhydrophobic Cellulose Nanofiber-Assembled Aerogels for Highly Efficient Water-in-Oil Emulsions Separation. ACS Appl. Nano Mater. 2018, 1, 2095–2103. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-Assembling Behavior of Cellulose Nanoparticles during Freeze-Drying: Effect of Suspension Concentration, Particle Size, Crystal Structure, and Surface Charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Alexanderson, J. Relations between structure and mechanical properties of autoclaved aerated concrete. Cem. Concr. Res. 1979, 9, 507–514. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, H.-B.; Liu, Z.; Xu, S.; Wang, Y.-Z. On controlling aerogel microstructure by freeze casting. Compos. Part B Eng. 2019, 173, 107036. [Google Scholar] [CrossRef]
- Liu, H.; Cao, C.; Wei, F.-F.; Huang, P.-P.; Sun, Y.-B.; Jiang, L.; Song, W. Flexible macroporous carbon nanofiber film with high oil adsorption capacity. J. Mater. Chem. A 2014, 2, 3557–3562. [Google Scholar] [CrossRef]
- Shi, G.; Qian, Y.; Tan, F.; Cai, W.; Li, Y.; Cao, Y. Controllable synthesis of pomelo peel-based aerogel and its application in adsorption of oil/organic pollutants. R. Soc. Open Sci. 2019, 6, 181823. [Google Scholar] [CrossRef] [Green Version]
- Sehaqui, H.; Zhou, Q.; Berglund, L.A. High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos. Sci. Technol. 2011, 71, 1593–1599. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, X.; Zhao, J.; Li, Q.; Ao, C.; Xia, T.; Zhang, W.; Lu, C. Ultra-lightweight and highly porous carbon aerogels from bamboo pulp fibers as an effective sorbent for water treatment. Results Phys. 2017, 7, 2919–2924. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, C.M.; Kafle, K. Characterization of crystalline cellulose in biomass: Basic principles, applications, and limitations of XRD, NMR, IR, Raman, and SFG. Korean J. Chem. Eng. 2013, 30, 2127–2141. [Google Scholar] [CrossRef]






| Aerogel | CNC/CNF Ratio | Density (g/cm3) | Porosity (%) |
|---|---|---|---|
| A-C/F11 | 1:1 | 0.0277 ± 0.004 | 98.027 ± 0.0024 |
| A-C/F12 | 1:2 | 0.0275 ± 0.002 | 98.364 ± 0.0002 |
| A-C/F13 | 1:3 | 0.0364 ± 0.0007 | 97.843 ± 0.0004 |
| A-C/F21 | 2:1 | 0.0227 ± 0.0009 | 98.659 ± 0.0006 |
| A-C/F31 | 3:1 | 0.0225 ± 0.0009 | 98.667 ± 0.0005 |
| Aerogel | CNC/CNF Ratio | Relative Pressure [P/Po] | Volume @ STP [cc/g] | 1/[W ((P/Po) − 1)] | Specific Surface Area (m2/g) |
|---|---|---|---|---|---|
| A-C/F11 | 1:1 | 0.2951 | 41.2105 | 8.13 | 126.42 |
| A-C/F12 | 1:2 | 0.3017 | 48.7459 | 7.09 | 148.15 |
| A-C/F13 | 1:3 | 0.2972 | 41.8069 | 8.094 | 202.72 |
| A-C/F21 | 2:1 | 0.3022 | 75.5596 | 4.587 | 464.39 |
| A-C/F31 | 3:1 | 0.2976 | 38.5557 | 29.54 | 117.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burhani, D.; Septevani, A.A.; Setiawan, R.; Djannah, L.M.; Putra, M.A.; Kusumah, S.S.; Sondari, D. Self-Assembled Behavior of Ultralightweight Aerogel from a Mixture of CNC/CNF from Oil Palm Empty Fruit Bunches. Polymers 2021, 13, 2649. https://doi.org/10.3390/polym13162649
Burhani D, Septevani AA, Setiawan R, Djannah LM, Putra MA, Kusumah SS, Sondari D. Self-Assembled Behavior of Ultralightweight Aerogel from a Mixture of CNC/CNF from Oil Palm Empty Fruit Bunches. Polymers. 2021; 13(16):2649. https://doi.org/10.3390/polym13162649
Chicago/Turabian StyleBurhani, Dian, Athanasia Amanda Septevani, Ruby Setiawan, Luthfia Miftahul Djannah, Muhammad Andrew Putra, Sukma Surya Kusumah, and Dewi Sondari. 2021. "Self-Assembled Behavior of Ultralightweight Aerogel from a Mixture of CNC/CNF from Oil Palm Empty Fruit Bunches" Polymers 13, no. 16: 2649. https://doi.org/10.3390/polym13162649