Bio-Based Alternatives to Phenol and Formaldehyde for the Production of Resins
Abstract
:1. Introduction
2. Materials and Methods
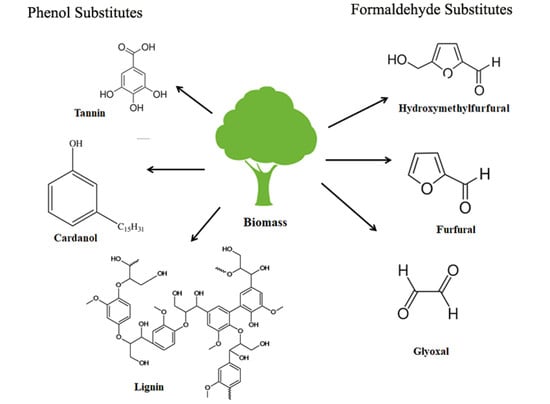
3. Phenol Substitutes for PF Resin
3.1. Lignin-Based PF Resins
3.2. Tannin-Based PF Resins
3.3. Cardanol-Based Phenolic Resin
4. Formaldehyde Substitutes for PF Resin
4.1. Hydroxymethylfurfural-Substituted Resin
4.2. Furfural-Based Resin
4.3. Glyoxal-Based Resin
5. Technical and Economic Challenges of Bio-Based Phenolic Resin
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Guo, L.; Zhang, H.; Zhai, H.; Ren, H. Research status, industrial application demand and prospects of phenolic resin. RSC Adv. 2019, 9, 28924–28935. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Zhang, X.-L. Application and development trend of phenolic resin. Thermosetting Resin 2007, 22, 47–50. [Google Scholar]
- Hirano, K.; Asami, M. Phenolic resins—100 years of progress and their future. React. Funct. Polym. 2013, 73, 256–269. [Google Scholar] [CrossRef]
- Natali, M.; Monti, M.; Kenny, J.M.; Torre, L. Synthesis and thermal characterization of phenolic resin/silica nanocomposites prepared with high shear rate-mixing technique. J. Appl. Polym. Sci. 2011, 120, 2632–2640. [Google Scholar] [CrossRef]
- Kim, Y.; Kamio, S.; Tajiri, T.; Hayashi, T.; Song, S.M.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Enhanced thermal conductivity of carbon fiber/phenolic resin composites by the introduction of carbon nanotubes. Appl. Phys. Lett. 2007, 90, 093125. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lv, R.; Huang, Z.; Liu, P.; Cong, P.; Li, T. Synthesis and characterization of a fluorinated phenolic resin/phenolic resin blend. J. Macromol. Sci. Part B 2016, 55, 85–98. [Google Scholar] [CrossRef]
- Abdalla, M.O.; Ludwick, A.; Mitchell, T. Boron-modified phenolic resins for high performance applications. Polymer 2003, 44, 7353–7359. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, N.; Zhang, T.; Li, T. Thermal stability and thermal degradation study of phenolic resin modified by cardanol. Emerg. Mater. Res. 2020, 9, 1–6. [Google Scholar] [CrossRef]
- Knop, A.; Pilato, L.A. Phenolic Resins: Chemistry, Applications and Performance; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Kamm, B. Production of platform chemicals and synthesis gas from biomass. Angew. Chem. Int. Ed. 2007, 46, 5056–5058. [Google Scholar] [CrossRef]
- Dashtban, M.; Gilbert, A.; Fatehi, P. Production of furfural: Overview and challenges. J. Sci. Technol. For. Prod. Process. 2012, 2, 44–53. [Google Scholar]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Fitzpatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef] [PubMed]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Harmsen, P.; Huijgen, W.; Bermudez, L.; Bakker, R. Literature Review of Physical and Chemical Pretreatment Processes for Lignocellulosic Biomass; Wageningen UR-Food & Biobased Research: Wageningen, The Netherlands, 2010. [Google Scholar]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzym. Res. 2011, 2011, 1–17. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, X.; Yang, G.; Li, Q.; Zhou, N. Preparation and characterization of a nanolignin phenol formaldehyde resin by replacing phenol partially with lignin nanoparticles. RSC Adv. 2019, 9, 29255–29262. [Google Scholar] [CrossRef]
- Ghorbani, M.; Liebner, F.; Pfungen, L.; Krahofer, M.; Budjav, E.; Konnerth, J.; Van Herwijnen, H.W.G. Lignin phenol formaldehyde resoles: The impact of lignin type on adhesive properties. BioResources 2016, 11, 6727–6741. [Google Scholar] [CrossRef] [Green Version]
- Solt, P.; Rößiger, B.; Konnerth, J.; Van Herwijnen, H.W.G. Lignin phenol formaldehyde resoles using base-catalysed depolymerized Kraft lignin. Polymers 2018, 10, 1162. [Google Scholar] [CrossRef] [Green Version]
- Manjula, S.; Pavithran, C.; Pillai, C.K.S.; Kumar, V.G. Synthesis and mechanical properties of cardanol-formaldehyde (CF) resins and CF-poly(methylmethacrylate) semi-interpenetrating polymer networks. J. Mater. Sci. 1991, 26, 4001–4007. [Google Scholar] [CrossRef]
- Li, J.; Zhu, W.; Zhang, J.; Gao, Q.; Xia, C.; Zhang, W.; Li, J. Depolymerization and characterization of Acacia mangium tannin for the preparation of mussel-inspired fast-curing tannin-based phenolic resins. Chem. Eng. J. 2019, 370, 420–431. [Google Scholar] [CrossRef]
- Tahir, P.M.; Halip, J.A.; Lee, S.H. Tannin-Based Bioresin as Adhesives. In Lignocellulose for Future Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–133. [Google Scholar]
- Shukor, N.F. Gallic Acid as a Potential Substitution for Phenol in Phenol-formaldehyde Resin for Biocomposite Matrices; University of Sheffield: Sheffield, UK, 2019. [Google Scholar]
- Dong, F.; Wang, M.; Wang, Z. Bio-oil as substitute of phenol for synthesis of resol-type phenolic resin as wood adhesive. Int. J. Chem. React. Eng. 2017, 16, 1–8. [Google Scholar] [CrossRef]
- Cui, Y.; Hou, X.; Wang, W.; Chang, J. Synthesis and characterization of bio-oil phenol formaldehyde resin used to fabricate phenolic based materials. Materials 2017, 10, 668. [Google Scholar] [CrossRef] [Green Version]
- Sui, G.; Cheng, Y.; Yang, X.; Wang, X.; Wang, Z. Use of sustainable glucose and furfural in the synthesis of formaldehyde-free phenolic resole resins. J. Appl. Polym. Sci. 2019, 136, 47733. [Google Scholar] [CrossRef]
- Foyer, G.; Chanfi, B.-H.; Virieux, D.; David, G.; Caillol, S. Aromatic dialdehyde precursors from lignin derivatives for the synthesis of formaldehyde-free and high char yield phenolic resins. Eur. Polym. J. 2016, 77, 65–74. [Google Scholar] [CrossRef]
- Ballerini, A.; Despres, A.; Pizzi, A. Non-toxic, zero emission tannin-glyoxal adhesives for wood panels. Holz als Roh-und Werkstoff 2005, 63, 477–478. [Google Scholar] [CrossRef]
- Kerns, W.D.; Pavkov, K.L.; Donofrio, D.J.; Gralla, E.J.; Swenberg, J.A. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 1983, 43, 4382–4392. [Google Scholar] [PubMed]
- Regulation (EC). 1272/2008,‘Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006’. Off. J. Eur. Union 2008, 50, 353. [Google Scholar]
- Asim, M.; Saba, N.; Jawaid, M.; Nasir, M.; Pervaiz, M.; Alothman, O.Y. A review on phenolic resin and its composites. Curr. Anal. Chem. 2018, 14, 185–197. [Google Scholar] [CrossRef]
- Tejado, A.; Pena, C.; Labidi, J.; Echeverria, J.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Trosa, A.; Pizzi, A. A no-aldehyde emission hardener for tannin-based wood adhesives for exterior panels. Holz als Roh-und Werkstoff 2001, 59, 266–271. [Google Scholar] [CrossRef]
- Devi, A.; Srivastava, D. Cardanol-based novolac-type phenolic resins. I. A kinetic approach. J. Appl. Polym. Sci. 2006, 102, 2730–2737. [Google Scholar] [CrossRef]
- Desch, H.E.; Dinwoodie, J.M. Timber: Structure, Properties, Conversion and Use; Macmillan International Higher Education: London, UK, 2016. [Google Scholar]
- Liu, C.; Hu, J.; Zhang, H.; Xiao, R. Thermal conversion of lignin to phenols: Relevance between chemical structure and pyrolysis behaviors. Fuel 2016, 182, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Lou, W.; Wang, L.; Yin, B.; Li, X. [C4H8SO3Hmim] HSO4 as an efficient catalyst for direct liquefaction of bagasse lignin: Decomposition properties of the inner structural units. Chem. Eng. Sci. 2015, 122, 24–33. [Google Scholar] [CrossRef]
- Shevchenko, S.M.; Paszner, L.; Saddler, J.N. Treatment of organosolv and steam explosion lignins with molecular hydrogen iodide: A structural probe. Cellul. Chem. Technol. 2001, 35, 295–318. [Google Scholar]
- Liu, Z.; Pang, H.; Jahan, M.S.; Ni, Y. Separation of lignocellulosic materials by combined processes of pre-hydrolysis and ethanol extraction. Bioresour. Technol. 2011, 102, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Pan, X.; Wang, G.; Gleisner, R. Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour. Technol. 2009, 100, 2411–2418. [Google Scholar] [CrossRef]
- Alriols, M.G.; García, A.; Llano-Ponte, R.; Labidi, J. Combined organosolv and ultrafiltration lignocellulosic biorefinery process. Chem. Eng. J. 2010, 157, 113–120. [Google Scholar] [CrossRef]
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels. Bioprod. Biorefin. 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ohta, H.; Fukuoka, A. Conversion of lignocellulose into renewable chemicals by heterogeneous catalysis. Catal. Sci. Technol. 2012, 2, 869–883. [Google Scholar] [CrossRef] [Green Version]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- El Mansouri, N.-E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Stücker, A.; Schütt, F.; Saake, B.; Lehnen, R. Lignins from enzymatic hydrolysis and alkaline extraction of steam refined poplar wood: Utilization in lignin-phenol-formaldehyde resins. Ind. Crops Prod. 2016, 85, 300–308. [Google Scholar] [CrossRef]
- Alonso, M.V.; Oliet, M.; Rodrıguez, F.; Garcıa, J.; Gilarranz, M.; Rodrıguez, J. Modification of ammonium lignosulfonate by phenolation for use in phenolic resins. Bioresour. Technol. 2005, 96, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Dilling, P. Method for Methylolation of Lignin Materials. U.S. Patent 4,764,597, 16 August 1988. [Google Scholar]
- Jiang, X.; Liu, J.; Du, X.; Hu, Z.; Chang, H.-M.; Jameel, H. Phenolation to improve lignin reactivity toward thermosets application. ACS Sustain. Chem. Eng. 2018, 6, 5504–5512. [Google Scholar] [CrossRef]
- Joseph, M.; Erich, A. Oxidative Demethylation of Lignin. U.S. Patent 3,071,570, 1 January 1963. [Google Scholar]
- Vazquez, G.; González-Alvarez, J.; Freire, S.; Antorrena, G.; Freire, M.S. Effect of chemical modification of lignin on the gluebond performance of lignin-phenolic resins. Bioresour. Technol. 1997, 60, 191–198. [Google Scholar] [CrossRef]
- El Mansouri, N.E.; Yuan, Q.; Huang, F. Preparation and characterization of phenol-formaldehyde resins modified with alkaline rice straw lignin. BioResources 2018, 13, 8061–8075. [Google Scholar] [CrossRef]
- Akim, L.; Shevchenko, S.; Zarubin, M. 13 C NMR studies on lignins depolymerized with dry hydrogen iodide. Wood Sci. Technol. 1993, 27, 241–248. [Google Scholar] [CrossRef]
- Chung, H.; Washburn, N.R. Improved lignin polyurethane properties with lewis acid treatment. ACS Appl. Mater. Interfaces 2012, 4, 2840–2846. [Google Scholar] [CrossRef] [Green Version]
- WU, S.; ZHAN, H. Characteristics of demethylated wheat straw soda lignin and its utilization in lignin-based phenolic formaldehyde resins. Cellul. Chem. Technol. 2001, 35, 253–262. [Google Scholar]
- Li, J.; Zhang, J.; Zhang, J.; Gao, Q.; Li, J.; Zhang, W. Fast curing bio-based phenolic resins via lignin demethylated under mild reaction condition. Polymers 2017, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Podschun, J.; Saake, B.; Lehnen, R. Reactivity enhancement of organosolv lignin by phenolation for improved bio-based thermosets. Eur. Polym. J. 2015, 67, 1–11. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Labidi, J. Organosolv lignin depolymerization with different base catalysts. J. Chem. Technol. Biotechnol. 2012, 87, 1593–1599. [Google Scholar] [CrossRef]
- Rößiger, B.; Röver, R.; Unkelbach, G.; Pufky-Heinrich, D. Production of bio-phenols for industrial application: Scale-up of the base-catalyzed depolymerization of lignin. Green Sustain. Chem. 2017, 7, 193. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, F.; Xu, J. Hydrogenolysis of lignosulfonate into phenols over heterogeneous nickel catalysts. Chem. Commun. 2012, 48, 7019–7021. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Bouxin, F.P.; McVeigh, A.; Tran, F.; Westwood, N.J.; Jarvis, M.C.; Jackson, S.D. Catalytic depolymerisation of isolated lignins to fine chemicals using a Pt/alumina catalyst: Part 1—Impact of the lignin structure. Green Chem. 2015, 17, 1235–1242. [Google Scholar] [CrossRef] [Green Version]
- Dier, T.K.F.; Rauber, D.; Durneata, D.; Hempelmann, R.; Volmer, D. Sustainable electrochemical depolymerization of lignin in reusable ionic liquids. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, G.F.; Weber, C.C.; Gräsvik, J.; Welton, T.; Brandt, A.; Hallett, J.P. Mechanistic insights into lignin depolymerisation in acidic ionic liquids. Green Chem. 2016, 18, 5456–5465. [Google Scholar] [CrossRef]
- Gosselink, R.J.; Teunissen, W.; Van Dam, J.E.; De Jong, E.; Gellerstedt, G.; Scott, E.L.; Sanders, J.P. Lignin depolymerisation in supercritical carbon dioxide/acetone/water fluid for the production of aromatic chemicals. Bioresour. Technol. 2012, 106, 173–177. [Google Scholar] [CrossRef]
- Abdelwahab, N.A.; Nassar, M.A. Preparation, optimisation and characterisation of lignin phenol formaldehyde resin as wood adhesive. Pigment Resin Technol. 2011, 40, 169–174. [Google Scholar] [CrossRef]
- Tachon, N.; Benjelloun-Mlayah, B.; Delmas, M. Organosolv wheat straw lignin as a phenol substitute for green phenolic resins. BioResources 2016, 11, 5797–5815. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Leitch, M.; Xu, C. Synthesis of phenol–formaldehyde resol resins using organosolv pine lignins. Eur. Polym. J. 2009, 45, 3380–3388. [Google Scholar] [CrossRef]
- Kalami, S.; Arefmanesh, M.; Master, E.; Nejad, M. Replacing 100% of phenol in phenolic adhesive formulations with lignin. J. Appl. Polym. Sci. 2017, 134, 45124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ma, Y.; Wang, C.; Li, S.; Zhang, M.; Chu, F. Preparation and properties of lignin–phenol–formaldehyde resins based on different biorefinery residues of agricultural biomass. Ind. Crops Prod. 2013, 43, 326–333. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, X.; Zheng, Z. Preparation and characterization of phenol–formaldehyde adhesives modified with enzymatic hydrolysis lignin. Bioresour. Technol. 2010, 101, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Li, S.; Guo, G.; Han, S.; Ren, S.; Ma, Y. Synthesis and characterization of phenol-formaldehyde resin using enzymatic hydrolysis lignin. J. Ind. Eng. Chem. 2015, 21, 1417–1422. [Google Scholar] [CrossRef]
- Domínguez, J.; Oliet, M.; Alonso, M.; Rojo, E.; Rodriguez, F. Structural, thermal and rheological behavior of a bio-based phenolic resin in relation to a commercial resol resin. Ind. Crops Prod. 2013, 42, 308–314. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Fu, Y.; Chang, J. Synthesis and characterization of phenol–furfural resins using lignin modified by a low transition temperature mixture. RSC Adv. 2016, 6, 94588–94594. [Google Scholar] [CrossRef]
- Jing, Z.; Lihong, H.; Bingchuan, L.; Caiying, B.; Puyou, J.; Yonghong, Z. Preparation and characterization of novolac phenol-formaldehyde resins with enzymatic hydrolysis lignin. J. Taiwan Inst. Chem. Eng. 2015, 54, 178–182. [Google Scholar] [CrossRef]
- Tejado, A.; Kortaberria, G.; Pena, C.; Blanco, M.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Lignins for phenol replacement in novolac-type phenolic formulations. II. Flexural and compressive mechanical properties. J. Appl. Polym. Sci. 2008, 107, 159–165. [Google Scholar] [CrossRef]
- Tejado, A.; Kortaberria, G.; Pena, C.; Labidi, J.; Echeverria, J.; Mondragon, I. Isocyanate curing of novolac-type ligno-phenol-formaldehyde resins. Ind. Crops Prod. 2008, 27, 208–213. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resource; Elsevier: Amsterdam, The Netherlands, 2008; pp. 179–199. [Google Scholar]
- Li, C.; Wang, W.; Mu, Y.; Zhang, W. Structural properties and copolycondensation mechanism of valonea tannin-modified phenol-formaldehyde resin. J. Polym. Environ. 2018, 26, 1297–1309. [Google Scholar] [CrossRef]
- Chowdhury, A.; Sarkar, S.; Chowdhury, A.; Bardhan, S.; Mandal, P.; Chowdhury, M. Tea waste management: A case study from West Bengal, India. Indian J. Sci. Technol. 2016, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Kim, K.H.; Jeon, B.T.; Cheong, S.H.; Park, J.H.; Kim, D.H.; Kweon, H.J.; Moon, S.H. Antibacterial and antioxidant activities of tannins extracted from agricultural by-products. J. Med. Plants Res. 2012, 6, 3072–3079. [Google Scholar] [CrossRef]
- Chen, M.; Luo, J.; Shi, R.; Zhang, J.; Gao, Q.; Li, J. Improved adhesion performance of soy protein-based adhesives with a larch tannin-based resin. Polymers 2017, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- García, D.; Glasser, W.; Pizzi, A.; Lacoste, C.; Laborie, M.-P. Polyphenolic resins prepared with maritime pine bark tannin and bulky-aldehydes. Ind. Crops Prod. 2014, 62, 84–93. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, N.; Feng, M.W. Bark extractives-based phenol–formaldehyde resins from beetle-infested lodgepole pine. J. Adhes. Sci. Technol. 2013, 27, 2112–2126. [Google Scholar] [CrossRef]
- De Yuso, A.M.; Lagel, M.C.; Pizzi, A.; Fierro, V.; Celzard, A. Structure and properties of rigid foams derived from quebracho tannin. Mater. Des. 2014, 63, 208–212. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Wang, W.; Zhang, W.; Li, J. Reactivity of larch and valonia tannins in synthesis of tannin-formaldehyde resins. BioResources 2016, 11, 2256–2268. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, J.; Yi, Z.; Yang, H.; Zhao, B.; Zhang, W.; Li, J. Preparation and characterization of a novel environmentally friendly phenol–formaldehyde adhesive modified with tannin and urea. Int. J. Adhes. Adhes. 2016, 66, 26–32. [Google Scholar] [CrossRef]
- Jahanshaei, S.; Tabarsa, T.; Asghari, J. Eco-friendly tannin-phenol formaldehyde resin for producing wood composites. Pigment Resin Technol. 2012, 41, 296–301. [Google Scholar] [CrossRef]
- Lagel, M.; Pizzi, A.; Giovando, S.; Celzard, A. Development and characterisation of phenolic foams with phenol-formaldehyde-chestnut tannins resin. J. Renew. Mater. 2014, 2, 220–229. [Google Scholar] [CrossRef]
- Abdalla, S.; Pizzi, A.; Bahabri, F.; Ganash, A. Analysis of valonia oak (Quercus aegylops) acorn tannin and wood adhesives application. BioResources 2015, 10, 7165–7177. [Google Scholar] [CrossRef] [Green Version]
- Hafiz, N.L.M.; Tahir, P.M.; Hua, L.S.; Abidin, Z.Z.; Sabaruddin, F.A.; Yunus, N.Y.M.; Abdullah, U.H.; Khalil, H.A. Curing and thermal properties of co-polymerized tannin phenol–formaldehyde resin for bonding wood veneers. J. Mater. Res. Technol. 2020, 9, 6994–7001. [Google Scholar] [CrossRef]
- Zhang, A.; Li, J.; Zhang, S.; Mu, Y.; Zhang, W.; Li, J. Characterization and acid-catalysed depolymerization of condensed tannins derived from larch bark. RSC Adv. 2017, 7, 35135–35146. [Google Scholar] [CrossRef] [Green Version]
- Mulani, K.; Daniels, S.; Rajdeo, K.; Tambe, S.; Chavan, N. Tannin-aniline-formaldehyde resole resins for arsenic removal from contaminated water. Can. Chem. Trans. 2014, 2, 450. [Google Scholar]
- Kuruppu, K.; Karunanayake, L. Synthesis and Characterization of Tannin Based Porous Cation Exchange Resins from Cassia auriculata (Ranawara). Vidyodaya J. Sci. 2019, 22, 17–31. [Google Scholar]
- Yi, Z.; Wang, W.; Zhang, W.; Li, J. Preparation of tannin–formaldehyde–furfural resin with pretreatment of depolymerization of condensed tannin and ring opening of furfural. J. Adhes. Sci. Technol. 2016, 30, 947–959. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.; Zhang, J.; Gao, Q.; Zhang, W.; Li, J. Larch tannin-based rigid phenolic foam with high compressive strength, low friability, and low thermal conductivity reinforced by cork powder. Compos. Part B Eng. 2019, 156, 368–377. [Google Scholar] [CrossRef]
- Pena, C.; Martín, M.D.; Tejado, A.; Labidi, J.; Echeverría, J.M.; Mondragon, I. Curing of phenolic resins modified with chestnut tannin extract. J. Appl. Polym. Sci. 2006, 101, 2034–2039. [Google Scholar] [CrossRef]
- Lochab, B.; Shukla, S.; Varma, I.K. Naturally occurring phenolic sources: Monomers and polymers. RSC Adv. 2014, 4, 21712–21752. [Google Scholar] [CrossRef]
- Nair, C.R.; Bindu, R.L.; Joseph, V.C. Cyanate esters based on cardanol modified-phenol-formaldehyde resins: Syntheses and thermal characteristics. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 621–627. [Google Scholar] [CrossRef]
- Mahanwar, P.; Kale, D. Effect of cashew nut shell liquid (CNSL) on properties of phenolic resins. J. Appl. Polym. Sci. 1996, 61, 2107–2111. [Google Scholar] [CrossRef]
- Parameswaran, P.; Abraham, B.; Thachil, E.T. Cardanol-based Resol Phenolics–A Comparative Study. Prog. Rubber Plast. Recycl. Technol. 2010, 26, 31–50. [Google Scholar] [CrossRef]
- Liang, B.; Li, X.; Hu, L.; Bo, C.; Zhou, J.; Zhou, Y. Foaming resol resin modified with polyhydroxylated cardanol and its application to phenolic foams. Ind. Crops Prod. 2016, 80, 194–196. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Z.; Liu, Y.; Li, Y. Novel cardanol-containing boron-modified phenolic resin composites: Non-isothermal curing kinetics, thermal properties, and ablation mechanism. High Perform. Polym. 2017, 29, 279–288. [Google Scholar] [CrossRef]
- Natarajan, M.; Murugavel, S.C. Synthesis, spectral and thermal degradation kinetics of novolac resins derived from cardanol. High Perform. Polym. 2013, 25, 685–696. [Google Scholar] [CrossRef]
- Shukla, S.K.; Srivastava, D.; Srivastava, K. Synthesis, Spectral and Thermal Degradation Kinetics of the Epoxidized Resole Resin Derived from Cardanol. Adv. Polym. Technol. 2015, 34, 21469. [Google Scholar] [CrossRef]
- J Jadhav, N.L.; Sastry, S.K.C.; Pinjari, D.V. Energy efficient room temperature synthesis of cardanol-based novolac resin using acoustic cavitation. Ultrason. Sonochem. 2018, 42, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Srivastava, D. Mechanical, chemical, and curing characteristics of cardanol–furfural-based novolac resin for application in green coatings. J. Coat. Technol. Res. 2015, 12, 303–311. [Google Scholar] [CrossRef]
- Rahmawati, P.; Ramelan, A.H.; Marliyana, S.D.; Suharty, N.S.; Wahyuningsih, S. Synthesis of cardanol-based novolac resin from cashew nut shell liquid. J. Eng. Sci. 2019, 15, 23–33. [Google Scholar] [CrossRef]
- 3Songur, A.; Ozen, O.A.; Sarsilmaz, M. The toxic effects of formaldehyde on the nervous system. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin, Germany, 2010; pp. 105–118. [Google Scholar]
- Duong, A.; Steinmaus, C.; McHale, C.M.; Vaughan, C.P.; Zhang, L. Reproductive and developmental toxicity of formaldehyde: A systematic review. Mutat. Res. Rev. Mutat. Res. 2011, 728, 118–138. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Freeman, L.E.B.; Nakamura, J.; Hecht, S.S.; Vandenberg, J.J.; Smith, M.T.; Sonawane, B.R. Formaldehyde and leukemia: Epidemiology, potential mechanisms, and implications for risk assessment. Environ. Mol. Mutagenes. 2010, 51, 181–191. [Google Scholar] [CrossRef] [Green Version]
- RTECS. Canadian Centre Occupational Health and Safety. Registry of Toxic Effects of Chemical Substances. 2018. Available online: https://www.ccohs.ca/products/ (accessed on 24 March 2020).
- Zhang, L. Formaldehyde: Exposure, Toxicity and Health Effects; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Binetti, R.; Costamagna, F.M.; Marcello, I. Development of carcinogenicity classifications and evaluations: The case of formaldehyde. Annali-Istituto Superiore Di Sanita 2006, 42, 132. [Google Scholar]
- Health JSfO. Recommendation of occupational exposure limits. J. Occup. Health 2000, 42, 213–228. [Google Scholar] [CrossRef]
- Zhang, L.; Steinmaus, C.; Eastmond, D.A.; Xin, X.K.; Smith, M.T. Formaldehyde exposure and leukemia: A new meta-analysis and potential mechanisms. Mutat. Res. Rev. Mutat. Res. 2009, 681, 150–168. [Google Scholar] [CrossRef]
- Metzger, J.O. Production of liquid hydrocarbons from biomass. Angew. Chem. Int. Ed. 2006, 45, 696–698. [Google Scholar] [CrossRef]
- Abdulmalik, O.; Safo, M.K.; Chen, Q.; Yang, J.; Brugnara, C.; Ohene-Frempong, K.; Abraham, D.J.; Asakura, T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br. J. Haematol. 2005, 128, 552–561. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman; Alam, N.; Khalil, I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, R.-J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, R. Upgrading of Carbohydrates to the Biofuel Candidate 5-Ethoxymethylfurfural (EMF). Int. J. Chem. Eng. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liang, X.; Li, J.; Li, Q. Catalytic hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to biofuel 2, 5-dimethylfuran. Appl. Catal. A Gen. 2019, 576, 85–95. [Google Scholar] [CrossRef]
- Girisuta, B.; Heeres, H.J. Levulinic acid from biomass: Synthesis and applications. In Production of Platform Chemicals from Sustainable Resources; Springer: Berlin, Germany, 2017; pp. 143–169. [Google Scholar]
- Li, Q.; Xing, J. Production of 1, 4-Diacids (Succinic, Fumaric, and Malic) from Biomass. In Production of Platform Chemicals from Sustainable Resources; Springer: Berlin, Germany, 2017; pp. 231–262. [Google Scholar]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2, 5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Ståhlberg, T.; Fu, W.; Woodley, J.M.; Riisager, A. Synthesis of 5-(hydroxymethyl) furfural in ionic liquids: Paving the way to renewable chemicals. ChemSusChem 2011, 4, 451–458. [Google Scholar] [CrossRef]
- Kuster, B. 5-Hydroxymethylfurfural (HMF). A review focussing on its manufacture. Starch-Stärke 1990, 42, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Brown, H.M.; Huang, X.; Zhou, X.-D.; Amonette, J.E.; Zhang, Z.C. Single-step conversion of cellulose to 5-hydroxymethylfurfural (HMF), a versatile platform chemical. Appl. Catal. A Gen. 2009, 361, 117–122. [Google Scholar] [CrossRef]
- Li, C.; Wang, Q.; Zhao, Z.K. Acid in ionic liquid: An efficient system for hydrolysis of lignocellulose. Green Chem. 2008, 10, 177–182. [Google Scholar] [CrossRef]
- Lange, J.P. Lignocellulose conversion: An introduction to chemistry, process and economics. Biofuels Bioprod. Biorefin. 2007, 1, 39–48. [Google Scholar] [CrossRef]
- Heo, J.B.; Lee, Y.-S.; Chung, C.-H. Raw plant-based biorefinery: A new paradigm shift towards biotechnological approach to sustainable manufacturing of HMF. Biotechnol. Adv. 2019, 37, 107422. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; De, S.; Saha, B. Advances in biomass transformation to 5-hydroxymethylfurfural and mechanistic aspects. Biomass Bioenergy 2013, 55, 355–369. [Google Scholar] [CrossRef]
- Zhang, Y.; Nanda, M.; Tymchyshyn, M.; Yuan, Z.; Xu, C. Mechanical, thermal, and curing characteristics of renewable phenol-hydroxymethylfurfural resin for application in bio-composites. J. Mater. Sci. 2016, 51, 732–738. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Z.; Mahmood, N.; Huang, S.; Xu, C. Sustainable bio-phenol-hydroxymethylfurfural resins using phenolated de-polymerized hydrolysis lignin and their application in bio-composites. Ind. Crops Prod. 2016, 79, 84–90. [Google Scholar] [CrossRef]
- Lytle, C.; Bertsch, W.; McKinley, M. Determination of novolac resin thermal decomposition products by pyrolysis-gas chromatography-mass spectrometry. J. Anal. Appl. Pyrolysis 1998, 45, 121–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Z.; Xu, C. Engineering biomass into formaldehyde-free phenolic resin for composite materials. AIChE J. 2015, 61, 1275–1283. [Google Scholar] [CrossRef]
- Zhang, Y.; Ferdosian, F.; Yuan, Z.; Xu, C. Sustainable glucose-based phenolic resin and its curing with a DGEBA epoxy resin. J. Taiwan Inst. Chem. Eng. 2017, 71, 381–387. [Google Scholar] [CrossRef]
- Lange, J.-P.; Van Der Heide, E.; Van Buijtenen, J.; Price, R. Furfural—A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Pizzi, A.; Orovan, E.; Cameron, F.A. The development of weather-and boil-proof phenol-resorcinol-furfural cold-setting adhesives. Holz als Roh-und Werkstoff 1984, 42, 467–472. [Google Scholar] [CrossRef]
- Patel, R.D.; Patel, R.G.; Patel, V.S.; Pearce, E.M. Kinetic investigation on the curing of phenol-furfural resin by differential scanning calorimetry. J. Appl. Polym. Sci. 1987, 34, 2583–2589. [Google Scholar] [CrossRef]
- Pizzi, A.; Pasch, H.; Simon, C.; Rode, K. Structure of resorcinol, phenol, and furan resins by MALDI-TOF mass spectrometry and 13C NMR. J. Appl. Polym. Sci. 2004, 92, 2665–2674. [Google Scholar] [CrossRef]
- Pizzi, A. Advanced Wood Adhesives Technology; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Oliveira, F.B.; Gardrat, C.; Enjalbal, C.; Frollini, E.; Castellan, A. Phenol–furfural resins to elaborate composites reinforced with sisal fibers—Molecular analysis of resin and properties of composites. J. Appl. Polym. Sci. 2008, 109, 2291–2303. [Google Scholar] [CrossRef]
- Cheng, Y.; Sui, G.; Liu, H.; Wang, X.; Yang, X.; Wang, Z. Preparation of highly phenol substituted bio-oil–phenol–formaldehyde adhesives with enhanced bonding performance using furfural as crosslinking agent. J. Appl. Polym. Sci. 2019, 136, 46995. [Google Scholar] [CrossRef]
- Koley, R.; Kasilingam, R.; Sahoo, S.; Chattopadhyay, S.; Bhowmick, A.K. Synthesis and Characterization of Phenol Furfural Resin from Moringa Oleifera Gum and Biophenol and Its Application in Styrene Butadiene Rubber. Ind. Eng. Chem. Res. 2019, 58, 18519–18532. [Google Scholar] [CrossRef]
- Ahuja, S.; Singh, D. A Kinetic Model of Alkali Catalyzed Phenol-furfural Novalac Resinification. Polym. Polym. Compos. 2011, 19, 581–586. [Google Scholar] [CrossRef]
- Dongre, P.; Driscoll, M.; Amidon, T.E.; Bujanovic, B. Lignin-furfural based adhesives. Energies 2015, 8, 7897–7914. [Google Scholar] [CrossRef] [Green Version]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344, 109–116. [Google Scholar] [CrossRef]
- Manini, P.; La Pietra, P.; Panzella, L.; Napolitano, A.; D’Ischia, M. Glyoxal formation by Fenton-induced degradation of carbohydrates and related compounds. Carbohydr. Res. 2006, 341, 1828–1833. [Google Scholar] [CrossRef]
- Hirayama, T.; Yamada, N.; Nohara, M.; Fukui, S. The high performance liquid chromatographic determination of total malondialdehyde in vegetable oil with dansyl hydrazine. J. Sci. Food Agric. 1984, 35, 338–344. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A. Some of physical and mechanical properties of particleboard panels bonded with phenol-lignin-glyoxal resin. J. Adhes. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H. Ionic liquid modified lignin-phenol-glyoxal resin: A green alternative resin for production of particleboards. J. Adhes. 2019, 95, 1075–1087. [Google Scholar] [CrossRef]
- Hussin, M.H.; Aziz, A.A.; Iqbal, A.; Ibrahim, M.N.M.; Latif, N.H.A. Development and characterization novel bio-adhesive for wood using kenaf core (Hibiscus cannabinus) lignin and glyoxal. Int. J. Biol. Macromol. 2019, 122, 713–722. [Google Scholar] [CrossRef]
- Aziz, N.A.; Latip, A.F.A.; Peng, L.C.; Latif, N.H.A.; Brosse, N.; Hashim, R.; Hussin, M.H. Reinforced lignin-phenol-glyoxal (LPG) wood adhesives from coconut husk. Int. J. Biol. Macromol. 2019, 141, 185–196. [Google Scholar] [CrossRef]
- Rao, G.-S.; Nabipour, H.; Zhang, P.; Hu, Y.; Xing, W.; Song, L.; Hu, Y. Lightweight, hydrophobic and recyclable carbon foam derived from lignin–resorcinol–glyoxal resin for oil and solvent spill capture. J. Mater. Res. Technol. 2020, 9, 4655–4664. [Google Scholar] [CrossRef]
- Pizzi, A.; Salvadó, J. Lignin-based wood panel adhesives without formaldehyde. Holz als Roh-und Werkstoff 2007, 65, 65. [Google Scholar]
- Ramires, E.C.; Megiatto, J.D.; Gardrat, C.; Castellan, A.; Frollini, E. Biobased composites from glyoxal–phenolic resins and sisal fibers. Bioresour. Technol. 2010, 101, 1998–2006. [Google Scholar] [CrossRef]
- Ang, A.F.; Ashaari, Z.; Bakar, E.S.; Ibrahim, N.A. Possibility of enhancing the dimensional stability of jelutong (Dyera costulata) wood using glyoxalated alkali lignin-phenolic resin as bulking agent. Eur. J. Wood Wood Prod. 2018, 76, 269–282. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, Y.; Xu, C.C. Synthesis and thermomechanical property study of Novolac phenol-hydroxymethyl furfural (PHMF) resin. RSC Adv. 2014, 4, 31829–31835. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; Lopes, A.M.D.C.; Lukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Roumeas, L.; Aouf, C.; Dubreucq, É.; Fulcrand, H. Depolymerisation of condensed tannins in ethanol as a gateway to biosourced phenolic synthons. Green Chem. 2013, 15, 3268–3275. [Google Scholar] [CrossRef]
- Chio, C.; Mohini, S.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]







| Substitute | PF Resin Type | % Substitution | Effect on Performance | Ref. |
|---|---|---|---|---|
| Kraft Lignin | Resol | 90 | Increase in adhesive strength and decrease in gel time | [67] |
| Organosolv wheat straw lignin | Resol | 50–70 | Resin achieved the specification of PF resin in terms of pH, viscosity, and solid content | [68] |
| Organosolv pine lignin | Resol | 25–75 | Substitution up to 50% decreases the curing temperature of the resin | [69] |
| Corn stover lignin | Resol | 100 | Mechanical properties similar to PF resin | [70] |
| Methylolated softwood ammonium lignosulfonate | Resol | 30 | High thermal stability and modified rheology | [74] |
| Enzymatic hydrolysis lignin | Novolac | 55 | Low free phenol and longer gelation time | [76] |
| Kraft pine lignin, Soda/anthraquinone flax lignin, and Sulfonated kraft lignin | Novolac | 25–45 | Increase in flexural modulus | [77] |
| Kraft lignin | Novolac | 25–45 | Low gelation time | [78] |
| Substitute | PF Resin Type | % Substitution | Effect on Performance | Ref. |
|---|---|---|---|---|
| Valonea Tannin | Resol | 30 | Short curing time, good bonding strength and low formaldehyde emission | [81] |
| Tannin | Resol | 20 | Low formaldehyde emission and could meet the requirement of GB/T17657-2013 | [90] |
| Condensed tannin | Resol | 10–30 | Lower PH, higher viscosity and shorter gel time | [91] |
| Chestnut tannin | Resol | 30 | Increase in compressive strength | [92] |
| Larch Tannin | Resol | 30 | Friability and thermal conductivity increased with increase in tannin content | [99] |
| Chestnut tannin | Novolac | 4–40 | Short gelation time | [100] |
| Substitute | PF Resin Type | % Substitution | Effect on Performance | Ref. |
|---|---|---|---|---|
| Cashew nut shell liquid | Resol | 10–90 | Improvement in impact strength and electrical properties | [103] |
| Polyhydroxylated cardanol | Resol | 20 | Increased in compressive strength and flexural strength. | [105] |
| Cardanol | Resol | 100 | Thermal stability increased | [108] |
| Cardanol | Novolac | 100 | Reduction in reaction time | [109] |
| Cashew nut shell liquid | Novolac | 100 | Increase in thermal stability | [111] |
| Country | OEL (ppm) | Reference | |||||
|---|---|---|---|---|---|---|---|
| TWA | STEL | TLV | |||||
| Australia | 1 | 2 | [115] | ||||
| China | 0.4 | [116] | |||||
| Germany | 0.3 | [117] | |||||
| Japan | 0.1 | [118] | |||||
| South Africa | 1 | 2 | [116] | ||||
| United Kingdom | 2 | 2 | [116] | ||||
| United States | 0.75 | 2 | [119] | ||||
| Substitute | PF Resin Type | % Substitution | Performance | Ref. |
|---|---|---|---|---|
| Hydroxymethylfurfural | Novolac | 100 | Increase in tensile strength | [163] |
| Hydroxymethylfurfural | Novolac | 100 | High thermal stability and increased curing time | [137] |
| Hydroxymethylfurfural | Novolac | 100 | High tensile strength and glass transition temperature | [140] |
| Furfural | Resol | 100 | Increased in tack and the rheological properties of the rubber | [149] |
| Furfural | Resol | 100 | High bonding strength, thermal stability, and low free phenol content | [75] |
| Furfural | Resol | 5 to 15 | Wet tensile strength increased | [148] |
| Glyoxal | Resol | 100 | Good internal bond strength | [160] |
| Glyoxal | Resol | 100 | Curing time can be varied | [161] |
| Glyoxal | Resol | 100 | Greater mechanical strength and dimensional stability | [156] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Bio-Based Alternatives to Phenol and Formaldehyde for the Production of Resins. Polymers 2020, 12, 2237. https://doi.org/10.3390/polym12102237
Sarika PR, Nancarrow P, Khansaheb A, Ibrahim T. Bio-Based Alternatives to Phenol and Formaldehyde for the Production of Resins. Polymers. 2020; 12(10):2237. https://doi.org/10.3390/polym12102237
Chicago/Turabian StyleSarika, P. R., Paul Nancarrow, Abdulrahman Khansaheb, and Taleb Ibrahim. 2020. "Bio-Based Alternatives to Phenol and Formaldehyde for the Production of Resins" Polymers 12, no. 10: 2237. https://doi.org/10.3390/polym12102237