Polyurethane Recycling and Disposal: Methods and Prospects
Abstract
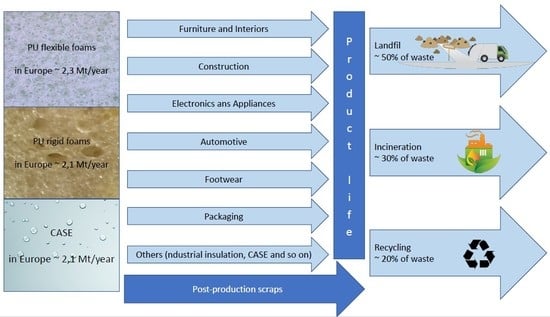
:1. Introduction
2. Polyurethanes
3. Polyurethane Waste Management
3.1. Landfilling
3.2. Mechanical Recycling
3.2.1. Mechanical Reprocessing with Adhesives
3.2.2. Mechanical Reprocessing without Adhesives
3.3. Chemical and Feedstock Recycling
3.3.1. Hydrolysis
3.3.2. Hydroglycolysis
3.3.3. Aminolysis/Ammonolysis
3.3.4. Phosphorolysis
3.3.5. Glycolysis
3.3.6. Gasification
3.3.7. Pyrolysis
3.3.8. Hydrogenation
3.4. Energy Recovery
3.5. Biological Degradation
3.5.1. Fungal Degradation
3.5.2. Bacterial Degradation
3.5.3. Enzymatic Degradation
3.5.4. Polyurethane Modification
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- PlasticsEurope Association of Plastics Manufacturers Plastics—The Facts 2019 An analysis of European Plastics Production, Demand and Waste Data. Available online: https://www.plasticseurope.org/en/resources/market-data (accessed on 25 July 2020).
- Magnin, A.; Pollet, E.; Phalip, V.; Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2020, 39, 107457. [Google Scholar] [CrossRef] [PubMed]
- IAL Consultants. The 12th Edition of Report on the Markets for Polyurethane Chemicals and Products in Europe, Middle East and Africa; IAL Consultants: Ealing, UK, 2018. [Google Scholar]
- Kurańska, M.; Prociak, A.; Kirpluks, M.; Cabulis, U. Polyurethane-polyisocyanurate foams modified with hydroxyl derivatives of rapeseed oil. Ind. Crops Prod. 2015, 74, 849–857. [Google Scholar] [CrossRef]
- Connolly, M.; King, J.; Shidaker, T.; Duncan, A. Pultruding Polyurethane Composite Profiles: Practical Guidelines for Injection Box Design, Component Metering Equipment and Processing. In Proceedings of the COMPOSITES 2005 Convention and Trade Show American Composites Manufacturers Association, Columbus, OH, USA, 28–30 September 2005; pp. 1–9. [Google Scholar]
- Babaarslan, O.; Sarıoğlu, E.; Ertek Avcı, M. A comparative study on performance characteristics of multicomponent core-spun yarns containing cotton/PET/elastane. J. Text. Inst. 2020, 111, 775–784. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications-a review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, S.; Kubota, M.; Lewis, R.D.; Advani, S.G.; Prasad, A.K.; Deitzel, J.M. Ultraviolet, water, and thermal aging studies of a waterborne polyurethane elastomer-based high reflectivity coating. Prog. Org. Coat. 2015, 79, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Howard, G.T. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Morton-Jones, D.H.; Ellis, J.W. Polymer Products, Design, Materials and Processing; Chapman and Hall Ltd.: London, UK, 1986; ISBN 9789401083201. [Google Scholar]
- Europeean Commission. Green Paper on a European Strategy on Plastic Waste in the Environment; Europeean Commission: Brussels, Belgium, 2013.
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Austin, A.; Hicks, D. A review of the global PU industry 2016 and outlook for 2017. PU Mag. 2017, 14, 4–16. [Google Scholar]
- European Diisocyanate & Polyol Producers Association. Available online: www.isopa.org (accessed on 3 July 2020).
- Gamerith, C.; Herrero Acero, E.; Pellis, A.; Ortner, A.; Vielnascher, R.; Luschnig, D.; Zartl, B.; Haernvall, K.; Zitzenbacher, S.; Strohmeier, G.; et al. Improving enzymatic polyurethane hydrolysis by tuning enzyme sorption. Polym. Degrad. Stab. 2016, 132, 69–77. [Google Scholar] [CrossRef]
- Garrido, M.A.; Font, R. Pyrolysis and combustion study of flexible polyurethane foam. J. Anal. Appl. Pyrolysis 2015, 113, 202–215. [Google Scholar] [CrossRef] [Green Version]
- Simón, D.; Borreguero, A.; de Lucas, A.; Gutiérrez, C.; Rodriguez, J. Sustainable Polyurethanes: Chemical Recycling to Get It. In Environment, Energy and Climate Change I; Jiménez, E., Cabañas, B., Lefebvre, G., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 5, pp. 1–12. ISBN 978-3-319-12907-5. [Google Scholar]
- Cregut, M.; Bedas, M.; Durand, M.J.; Thouand, G. New insights into polyurethane biodegradation and realistic prospects for the development of a sustainable waste recycling process. Biotechnol. Adv. 2013, 31, 1634–3647. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Off. J. Eur. Union 2008, 34, 99–126. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0098 (accessed on 5 August 2020).
- Datta, J.; Kopczyńska, P.; Simón, D.; Rodríguez, J.F. Thermo-Chemical Decomposition Study of Polyurethane Elastomer through Glycerolysis Route with Using Crude and Refined Glycerine as a Transesterification Agent. J. Polym. Environ. 2018, 26, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Datta, J.; Włoch, M. Recycling of Polyurethanes. In Polyurethane Polymers: Blends and Interpenetrating Polymer Networks; Sabu, T., Datta, J., Haponiuk, J., Arunima, R., Eds.; eBook; Elsevier: Amsterdam, The Netherlands, 2017; pp. 323–358. ISBN 9780128040850. [Google Scholar]
- Helsen, L.; Bosmans, A. Waste-to-Energy through thermochemical processes: Matching waste with process. In Proceedings of the International Academic Symposium on Enhanced Landfill Mining, Houthalen-Hechteren, Belgium, 4–6 October 2010. [Google Scholar]
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Nestle, D.E. Foam-Rebonding Method. U.S. Patent 5,292,462, 8 March 1994. [Google Scholar]
- Hulme, A.J.; Goodhead, T.C. Cost effective reprocessing of polyurethane by hot compression moulding. J. Mater. Process. Technol. 2003, 139, 322–326. [Google Scholar] [CrossRef]
- Bleys, G.J.; James, H.A.; Cassidy, E.F. Method for the Preparation of Polyurethane Elastomers. U.S. Patent 6,069,184 A, 30 May 2000. [Google Scholar]
- American Chemistry Council. Available online: http://polyurethane.americanchemistry.com (accessed on 3 July 2020).
- Gerlock, J.L.; Braslaw, J.; Mahoney, L.R.; Ferris, F.C. Reaction of Polyurethane Foam with Dry Steam: Kinetics and Mechanism of Reactions. J. Polym. Sci. A1 1980, 18, 541–557. [Google Scholar] [CrossRef]
- Mahoney, L.R.; Weiner, S.A.; Ferris, F.C. Hydrolysis of Polyurethane Foam Waste. Environ. Sci. Technol. 1974, 8, 135–139. [Google Scholar] [CrossRef]
- Alavi Nikje, M.M.; Nikrah, M.; Mohammadi, F.H.A. Microwave-assisted polyurethane bond cleavage via hydroglycolysis process at atmospheric pressure. J. Cell. Plast. 2008, 44, 367–380. [Google Scholar] [CrossRef]
- Sheratte, M.B. Process for Converting the Decomposition Products of Polyurethane and Novel Compositions Thereby Obtained. U.S. Patent 4,110,266, 29 August 1978. [Google Scholar]
- Bauer, M.; Jörg, B.; Göcks, K. Method of Decomposing Polycyanurate-Containing Materials. U.S. Patent 5,691,388, 25 November 1997. [Google Scholar]
- Troev, K.; Grancharov, G.; Tsevi, R.; Tsekova, A. A novel approach to recycling of polyurethanes: Chemical degradation of flexible polyurethane foams by triethyl phosphate. Polymer 2000, 41, 7017–7022. [Google Scholar] [CrossRef]
- Zevenhoven, R. Treatment and disposal of polyurethane wastes: Options for recovery and recycling. In Energy Eng. Environ. Prot. Publ. Espoo 2004. Rep. TKK-ENY-19; Helsinki University of Technology: Helsinki, Finland, 2004. [Google Scholar]
- Simón, D.; Borreguero, A.M.; De Lucas, A.; Rodríguez, J.F. Glycolysis of viscoelastic flexible polyurethane foam wastes. Polym. Degrad. Stab. 2015, 116, 23–35. [Google Scholar] [CrossRef]
- Modesti, M.; Simioni, F.; Munari, R.; Baldoin, N. Recycling of flexible polyurethane foams with a low aromatic amine content. React. Funct. Polym. 1995, 26, 157–165. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, W.; Wang, L.; Hao, J. Comparative study of nitrogen migration among the products from catalytic pyrolysis and gasification of waste rigid polyurethane foam. J. Anal. Appl. Pyrolysis 2016, 120, 144–153. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Jomaa, G.; Goblet, P.; Coquelet, C.; Morlot, V. Kinetic modeling of polyurethane pyrolysis using non-isothermal thermogravimetric analysis. Thermochim. Acta 2015, 612, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Font, R.; Fullana, A.; Caballero, J.A.; Candela, J.; García, A. Pyrolysis study of polyurethane. J. Anal. Appl. Pyrolysis 2001, 58–59, 63–77. [Google Scholar] [CrossRef]
- Paabo, M.; Levin, B.C. A review of the literature on the gaseous products and toxicity generated from the pyrolysis and combustion of rigid polyurethane foams. Fire Mater. 1987, 11, 1–29. [Google Scholar] [CrossRef]
- Garrido, M.A.; Gerecke, A.C.; Heeb, N.; Font, R.; Conesa, J.A. Isocyanate emissions from pyrolysis of mattresses containing polyurethane foam. Chemosphere 2017, 168, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Garrido, M.A.; Font, R.; Conesa, J.A. Pollutant emissions from the pyrolysis and combustion of viscoelastic memory foam. Sci. Total Environ. 2017, 577, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Oceguera-Cervantes, A.; Carrillo-García, A.; López, N.; Bolaños-Nuñez, S.; Cruz-Gómez, M.J.; Wacher, C.; Loza-Tavera, H. Characterization of the polyurethanolytic activity of two Alicycliphilus sp. strains able to degrade polyurethane and N-methylpyrrolidone. Appl. Environ. Microbiol. 2007, 73, 6214–6223. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.H.M.M.; Mahmoud, W.M.; Davis, E.M.; Coughlin, R.W. Biodegradation of polyurethane coatings by hydrocarbon-degrading bacteria. Int. Biodeterior. Biodegrad. 1996, 37, 69–79. [Google Scholar] [CrossRef]
- Howard, G.T.; Norton, W.N.; Burks, T. Growth of Acinetobacter gerneri P7 on polyurethane and the purification and characterization of a polyurethanase enzyme. Biodegradation 2012, 23, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.; Howard, G.T. Growth of Bacillus subtilis on polyurethane and the purification and characterization of a polyurethanase-lipase enzyme. Int. Biodeterior. Biodegrad. 2002, 50, 33–40. [Google Scholar] [CrossRef]
- Nakkabi, A.; Sadiki, M.; Fahim, M.; Ittobane, N.; Ibnsouda Koraichi, S.; Barkai, H.; El abed, S. Biodegradation of Poly(ester urethane)s by Bacillus subtilis. Int. J. Environ. Res. 2015, 9, 157–162. [Google Scholar] [CrossRef]
- Nair, S.; Kumar, P. Molecular characterization of a lipase-producing Bacillus pumilus strain (NMSN-1d) utilizing colloidal water-dispersible polyurethane. World J. Microbiol. Biotechnol. 2007, 23, 1441–1449. [Google Scholar] [CrossRef]
- Allen, A.B.; Hilliard, N.P.; Howard, G.T. Purification and characterization of a soluble polyurethane degrading enzyme from Comamonas acidovorans. Int. Biodeterior. Biodegrad. 1999, 43, 37–41. [Google Scholar] [CrossRef]
- Howard, G.T.; Vicknair, J.; Mackie, R.I. Sensitive plate assay for screening and detection of bacterial polyurethanase activity. Lett. Appl. Microbiol. 2001, 32, 211–214. [Google Scholar] [CrossRef]
- Ruiz, C.; Howard, G.T. Nucleotide sequencing of a polyurethanase gene (pulA) from Pseudomonas fluorescens. Int. Biodeterior. Biodegrad. 1999, 44, 127–131. [Google Scholar] [CrossRef]
- Peng, Y.H.; Shih, Y.H.; Lai, Y.C.; Liu, Y.Z.; Liu, Y.T.; Lin, N.C. Degradation of polyurethane by bacterium isolated from soil and assessment of polyurethanolytic activity of a Pseudomonas putida strain. Environ. Sci. Pollut. Res. 2014, 21. [Google Scholar] [CrossRef]
- Crabbe, J.R.; Campbell, J.R.; Thompson, L.; Walz, S.L.; Schultz, W.W. Biodegradation of a colloidal ester-based polyurethane by soil fungi. Int. Biodeterior. Biodegrad. 1994, 33, 103–113. [Google Scholar] [CrossRef]
- Álvarez-Barragán, J.; Domínguez-Malfavón, L.; Vargas-Suárez, M.; González-Hernández, R.; Aguilar-Osorio, G.; Loza-Tavera, H. Biodegradative activities of selected environmental fungi on a polyester polyurethane varnish and polyether polyurethane foams. Appl. Environ. Microbiol. 2016, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, J.R.; Huang, J.; Anand, P.; Kucera, K.; Sandoval, A.G.; Dantzler, K.W.; Hickman, D.S.; Jee, J.; Kimovec, F.M.; Koppstein, D.; et al. Biodegradation of polyester polyurethane by endophytic fungi. Appl. Environ. Microbiol. 2011, 77, 6076–6084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Lara, L.F.; Vargas-Suárez, M.; Lõpez-Castillo, N.N.; Cruz-Gõmez, M.J.; Loza-Tavera, H. Preliminary study on the biodegradation of adipate/phthalate polyester polyurethanes of commercial-type by Alicycliphilus sp. BQ8. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Kay, M.J.; Morton, L.H.G.; Prince, E.L. Bacterial degradation of polyester polyurethane. Int. Biodeterior. 1991, 27, 205–222. [Google Scholar] [CrossRef]
- Gautam, R.; Bassi, A.S.; Yanful, E.K.; Cullen, E. Biodegradation of automotive waste polyester polyurethane foam using Pseudomonas chlororaphis ATCC55729. Int. Biodeterior. Biodegrad. 2007, 60, 245–249. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Akhter, J.I.; Hameed, A.; Ahmed, S. Degradation of polyurethane by novel bacterial consortium isolated from soil. Ann. Microbiol. 2008, 58, 381–386. [Google Scholar] [CrossRef]
- Akutsu, Y.; Nakajima-Kambe, T.; Nomura, N.; Nakahara, T. Purification and properties of a polyester polyurethane-degrading enzyme from Comamonas acidovorans TB-35. Appl. Environ. Microbiol. 1998, 64, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Nakajima-Kambe, T.; Onuma, F.; Akutsu, Y.; Nakahara, T. Determination of the polyester polyurethane breakdown products and distribution of the polyurethane degrading enzyme of Comamonas acidovorans strain TB-35. J. Ferment. Bioeng. 1997, 83, 456–460. [Google Scholar] [CrossRef]
- Nakajima-Kambe, T.; Onuma, F.; Kimpara, N.; Nakahara, T. Isolation and characterization of a bacterium which utilizes polyester polyurethane as a sole carbon and nitrogen source. FEMS Microbiol. Lett. 1995, 129, 39–42. [Google Scholar] [CrossRef]
- Fernandes, I.P.; Barbosa, M.; Amaral, J.S.; Pinto, V.; Rodrigues, J.L.; Ferreira, M.J.; Barreiro, M.F. Biobased additives as biodegradability enhancers with application in TPU-based footwear components. J. Renew. Mater. 2016, 4, 47–56. [Google Scholar] [CrossRef]
- Shah, Z.; Gulzar, M.; Hasan, F.; Shah, A.A. Degradation of polyester polyurethane by an indigenously developed consortium of Pseudomonas and Bacillus species isolated from soil. Polym. Degrad. Stab. 2016, 134. [Google Scholar] [CrossRef]
- Shah, Z.; Hasan, F.; Krumholz, L.; Atkas, D.; Shah, A.A. Degradation of polyester polyurethane by newly isolated Pseudomonas aeruginosa strain MZA-85 and analysis of degradation products by GC-MS. Int. Biodeterior. Biodegrad. 2013, 77, 114–122. [Google Scholar] [CrossRef]
- Magnin, A.; Hoornaert, L.; Pollet, E.; Laurichesse, S.; Phalip, V.; Avérous, L. Isolation and characterization of different promising fungi for biological waste management of polyurethanes. Microb. Biotechnol. 2018, 12, 544–555. [Google Scholar] [CrossRef]
- Ibrahim, I.N.; Maraqa, A.; Hameed, K.M.; Saadoun, I.M.; Maswadeh, H.M.; Nakajima-Kambe, T. Polyester-polyurethane biodegradation by Alternaria solani, isolated from northern Jordan. Adv. Environ. Biol. 2009, 3, 162–170. [Google Scholar]
- Mathur, G.; Prasad, R. Degradation of polyurethane by Aspergillus flavus (ITCC 6051) isolated from soil. Appl. Biochem. Biotechnol. 2012, 167, 1595–1602. [Google Scholar] [CrossRef]
- Osman, M.; Satti, S.M.; Luqman, A.; Hasan, F.; Shah, Z.; Shah, A.A. Degradation of Polyester Polyurethane by Aspergillus sp. Strain S45 Isolated from Soil. J. Polym. Environ. 2018, 26, 301–310. [Google Scholar] [CrossRef]
- Khan, S.; Nadir, S.; Shah, Z.U.; Shah, A.A.; Karunarathna, S.C.; Xu, J.; Khan, A.; Munir, S.; Hasan, F. Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ. Pollut. 2017, 225, 469–480. [Google Scholar] [CrossRef]
- Oprea, S.; Potolinca, V.O.; Gradinariu, P.; Joga, A.; Oprea, V. Synthesis, properties, and fungal degradation of castor-oil-based polyurethane composites with different cellulose contents. Cellulose 2016, 23, 2515–2526. [Google Scholar] [CrossRef]
- Shuttleworth, W.A.; Seal, K.J. A rapid technique for evaluating the biodeterioration potential of polyurethane elastomers. Appl. Microbiol. Biotechnol. 1986, 23, 407–409. [Google Scholar] [CrossRef]
- Jansen, B.; Schumacher-Perdreau, F.; Peters, G.; Pulverer, G. Evidence for Degradation of Synthetic Polyurethanes by Staphylococcus epidermidis. Zent. Bakteriol. 1991, 276, 36–45. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Murata, N.; Tanabe, E.; Kubota, K.; Kubo, M. Isolation and characterization of an ether-type polyurethane-degrading micro-organism and analysis of degradation mechanism by Alternaria sp. J. Appl. Microbiol. 2010, 108, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Filip, Z. Polyurethane as the Sole Nutrient Source for Aspergillus niger and Cladosporium herbarum. Eur. J. Appl. Microbiol. Biotechnol. 1979, 7, 277–280. [Google Scholar] [CrossRef]
- Howard, G.T.; Hilliard, N.P. Use of Coomassie blue-polyurethane interaction in screening of polyurethanase proteins and polyurethanolytic bacteria. Int. Biodeterior. Biodegrad. 1999, 43, 23–30. [Google Scholar] [CrossRef]
- Cosgrove, L.; McGeechan, P.L.; Robson, G.D.; Handley, P.S. Fungal communities associated with degradation of polyester polyurethane in soil. Appl. Environ. Microbiol. 2007, 73, 5817–5824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, R.; Bassi, A.S.; Yanful, E.K. Candida rugosa lipase-catalyzed polyurethane degradation in aqueous medium. Biotechnol. Lett. 2007, 29, 1081–1086. [Google Scholar] [CrossRef]
- Biffinger, J.C.; Barlow, D.E.; Cockrell, A.L.; Cusick, K.D.; Hervey, W.J.; Fitzgerald, L.A.; Nadeau, L.J.; Hung, C.S.; Crookes-Goodson, W.J.; Russell, J.N. The applicability of Impranil® DLN for gauging the biodegradation of polyurethanes. Polym. Degrad. Stab. 2015, 120, 178–185. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Perrin, R.; Ullmann, C.; Persillon, C.; Phalip, V.; Avérous, L. Enzymatic recycling of thermoplastic polyurethanes: Synergistic effect of an esterase and an amidase and recovery of building blocks. Waste Manag. 2019, 85, 141–150. [Google Scholar] [CrossRef]
- Schmidt, J.; Wei, R.; Oeser, T.; Dedavid e Silva, L.A.; Breite, D.; Schulze, A.; Zimmermann, W. Degradation of polyester polyurethane by bacterial polyester hydrolases. Polymers 2017, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- do Canto, V.P.; Thompson, C.E.; Netz, P.A. Polyurethanases: Three-dimensional structures and molecular dynamics simulations of enzymes that degrade polyurethane. J. Mol. Graph. Model. 2019, 89, 82–95. [Google Scholar] [CrossRef]
- Takamoto, T.; Shirasaka, H.; Uyama, H.; Kobayashi, S. Lipase-catatyzed hydrolytic degradation of polyurethane in organic solvent. Chem. Lett. 2001, 492–493. [Google Scholar] [CrossRef]
- Zhang, Z.; King, M.; Guidoin, R.; Therrien, M.; Doillon, C.; Diehl-Jones, W.L.; Huebner, E. In vitro exposure of a novel polyesterurethane graft to enzymes: A study of the biostability of the prosthesis. Biomaterials 1994, 15, 1129–1144. [Google Scholar] [CrossRef]
- Smith, R.; Williams, D.F.; Oliver, C. The biodegradation of poly (ether urethanes). J. Biomed. Mater. Res. 1987, 21, 1149–1166. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Gladhill, K.W.; Horbett, T.A. Analysis of in vitro enzymatic and oxidative degradation of polyurethanes. J. Biomed. Mater. Res. 1988, 22, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.; De Paz, M.V.; Zamora, F.; Galbis, J.A. Dithiothreitol-based polyurethanes. Synthesis and degradation studies. Polym. Degrad. Stab. 2010, 95, 1480–1487. [Google Scholar] [CrossRef]
- Mendoza-Novelo, B.; González-García, G.; Mata-Mata, J.L.; Castellano, L.E.; Cuéllar-Mata, P.; Ávila, E.E. A biological scaffold filled with silica and simultaneously crosslinked with polyurethane. Mater. Lett. 2013, 106, 369–372. [Google Scholar] [CrossRef]
- Tang, Y.W.; Labow, R.S.; Santerre, J.P. Enzyme-induced biodegradation of polycarbonate-polyurethanes: Dependence on hard-segment chemistry. J. Biomed. Mater. Res. 2001, 57, 597–611. [Google Scholar] [CrossRef]
- Tang, Y.W.; Labow, R.S.; Santerre, J.P. Isolation of methylene dianiline and aqueous-soluble biodegradation products from polycarbonate-polyurethanes. Biomaterials 2003, 24, 2805–2819. [Google Scholar] [CrossRef]
- Ciardelli, G.; Rechichi, A.; Cerrai, P.; Tricoli, M.; Barbani, N.; Giusti, P. Segmented polyurethanes for medical applications: Synthesis, characterization and in vitro enzymatic degradation studies. Macromol. Symp. 2004, 218, 261–272. [Google Scholar] [CrossRef]
- Fang, J.; Ye, S.H.; Shankarraman, V.; Huang, Y.; Mo, X.; Wagner, W.R. Biodegradable poly (ester urethane) urea elastomers with variable amino content for subsequent functionalization with phosphorylcholine. Acta Biomater. 2014, 10, 4639–4649. [Google Scholar] [CrossRef] [Green Version]
- Santerre, J.P.; Labow, R.S.; Adams, G.A. Enzyme–biomaterial interactions: Effect of biosystems on degradation of polyurethanes. J. Biomed. Mater. Res. 1993, 27, 97–109. [Google Scholar] [CrossRef]
- Santerre, J.P.; Labow, R.S.; Duguay, D.G.; Erfle, D.; Adams, G.A. Biodegradation evaluation of polyether and polyester-urethanes with oxidative and hydrolytic enzymes. J. Biomed. Mater. Res. 1994, 28, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.B.; Labow, R.S.; Santerre, J.P. Biodegradation of a poly (ester) urea-urethane by cholesterol esterase: Isolation and identification of principal biodegradation products. J. Biomed. Mater. Res. 1997, 36, 407–417. [Google Scholar] [CrossRef]
- Labow, R.S.; Erfle, D.J.; Santerre, J.P. Elastase-induced hvdrolvsis of synthetic solid sub & rate & poly (ester-urea-urethane) and poly(ether-urea-urethane). Biomaterials 1996, 17, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Marchant, R.E.; Anderson, J.M.; Hiltner, A. Long term biodegradation in vitro of poly(ether urethane urea): A mechanical property study. Polymer 1987, 28, 2040–2046. [Google Scholar] [CrossRef]
- Pilch-Pitera, B. Examination of the Enzyme Resistance of Polyurethane Powder Coatings. J. Polym. Environ. 2013, 21, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Rafiei, R.; Mohseni, M.; Yari, H.; Mahdavi, M. Evaluation of degradability of two polyurethane refinish coatings against biological materials: A case study. Prog. Org. Coat. 2016, 93, 1–10. [Google Scholar] [CrossRef]
- Mahajan, N.; Gupta, P. New insights into the microbial degradation of polyurethanes. RSC Adv. 2015, 5, 41839–41854. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethane; Rapra Technology Limited: Shawbury, UK, 2005; ISBN 978-1-84735-035-0. [Google Scholar]
- Phua, S.K.; Castillo, E.; Anderson, J.M.; Hiltner, A. Biodegradation of a polyurethane in vitro. J. Biomed. Mater. Res. 1987, 21, 231–246. [Google Scholar] [CrossRef]
- Pivec, T.; Sfiligoj-Smole, M.; Gašparič, P.; Stana-Kleinschek, K. Polyurethanes for Medical Use. Polyurethanes Med. Use 2017, 60. [Google Scholar] [CrossRef]
- Mizera, K.; Ryszkowska, J. Polyurethane elastomers from polyols based on soybean oil with a different molar ratio. Polym. Degrad. Stab. 2016, 132, 21–31. [Google Scholar] [CrossRef]
- Das, B.; Konwar, U.; Mandal, M.; Karak, N. Sunflower oil based biodegradable hyperbranched polyurethane as a thin film material. Ind. Crops Prod. 2013, 44, 396–404. [Google Scholar] [CrossRef]
- Spontón, M.; Casis, N.; Mazo, P.; Raud, B.; Simonetta, A.; Ríos, L.; Estenoz, D. Biodegradation study by Pseudomonas sp. of flexible polyurethane foams derived from castor oil. Int. Biodeterior. Biodegrad. 2013, 85, 85–94. [Google Scholar] [CrossRef]
- Ng, W.S.; Lee, C.S.; Chuah, C.H.; Cheng, S.F. Preparation and modification of water-blown porous biodegradable polyurethane foams with palm oil-based polyester polyol. Ind. Crops Prod. 2017, 97, 65–78. [Google Scholar] [CrossRef]
- Huang, G.; Wang, P. Effects of preparation conditions on properties of rigid polyurethane foam composites based on liquefied bagasse and jute fibre. Polym. Test. 2017, 60, 266–273. [Google Scholar] [CrossRef]
- Jašo, V.; Glenn, G.; Klamczynski, A.; Petrović, Z.S. Biodegradability study of polylactic acid/ thermoplastic polyurethane blends. Polym. Test. 2015, 47, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, N.; Nakayama, A.; Oshima, M.; Kawasaki, N.; Aiba, S.I. Enzymatic hydrolysis of lysine diisocyanate based polyurethanes and segmented polyurethane ureas by various proteases. React. Funct. Polym. 2007, 67, 1338–1345. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Pasa, V.M.D. Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Malewska, E.; Bąk, S.; Kurańska, M.; Prociak, A. The effect of various rapeseed oil-based polyols on selected properties of flexible polyurethane foams. Polimery 2016, 61, 799–806. [Google Scholar] [CrossRef]









| Rubber | Metal | Plastic |
|---|---|---|
| High abrasion resistance | Lightweight | High impact resistance |
| High cut and tear resistance | Noise reduction | Elastic memory |
| Superior load bearing | Abrasion resistance | Abrasion resistance |
| Thick section molding | Less expensive fabrication | Noise reduction |
| Colorability | Corrosion resistance | Variable coefficient of friction |
| Oil resistance | Resilience | Resilience |
| Ozone resistance | Impact resistance | Thick section molding |
| Radiation resistance | Flexibility | Lower cost tooling |
| Broader hardness range | Easily moldable | Low temperature resistance |
| Castable nature | Non-conductive | Cold flow resistance |
| Low pressure tooling | Non-sparking | Radiation resistance |
| Treatment | Input | Output | Large Scale Application |
|---|---|---|---|
| Hydrolysis | EOL products production scraps | polyols, amine intermediates | No |
| Hydroglycolysis | EOL products, production scraps | polyols | No |
| Aminolysis | only foams | bi- or polyfunctional amines and alcohols | No |
| Phosphorolysis | production scraps | phosphorus containing oligouretanes | No |
| Glycolysis | only foams, segregated for rigid and flexible | polyols | Yes |
| Gasification | EOL products, production scraps | syngas | Yes |
| Pyrolysis | EOL products, production scraps | oil, gas, ash | No |
| Hydrogenation | EOL products, production scraps | gas, oil | No |
| Microorganism Type | ||
|---|---|---|
| Bacteria | Fungi | |
| Polyester PU coating (including Impranil®) | Alicycliphilus sp. [46] Arthrobacter calcoaceticus [47] Acinetobacter garnei [48] Arthrobacter globiformis [47] Bacillus subtilis [49,50] Bacillus pumilus [51] Commamonas acidovorans [52] Pseudomonas aeruginosa [47] Pseudomonas cepacian [47] Pseudomonas chlororaphis [53] Pseudomonas fluorescens [54] Pseudomonas putida [47,55] | Aureobasidium pullulans [56] Cladosporium sp. [56] Cladosporium asperulatum [57] Curvularia senegalensis [56] Fusarium solani [56] Penicillium chrysogenum [57] Pestalotiopsis microspore [58] |
| Polyester PU foam | Alycycliphilus sp. [59] Pseudomonas aeruginosa [60] Pseudomonas chlororaphis [61] | |
| Thermoplastic polyester PU | Arthrobacter sp. [62] Bacillus sp. [62] Comamonas acidovorans [63,64,65] Corynebacterium [60] Micrococcus sp. [62] Pseudomonas sp. [62] Pseudomonas aeruginosa [62,66,67,68] | Alternaria sp., [69] Alternaria solani [70] Aspergillus flavus [71] Aspergillus fumigatus [72] Aspergillus section flavi [69] Aspergillus tubigensis [73] Chaetomium globosum [74] Gliocladium roseum [75] Penicillium sp. [69] |
| Thermoplastic polyether PU | Staphylococcus epidermidis [76] | |
| Polyether PU foam | Alternaria sp. [77] Aspergillus fumigatus [57] Cladosporium tenuissinum [57] Cladosporium asperulatum [57] Cladosporium herbarum [78] Cladosporium montecillanum [57] Cladosporium pseudocladosporoides [57] Penicillium chrysogenum [57] | |
| Type of PU | Enzyme |
|---|---|
| Polyester PU (Impranil) | Esterase [49,52,82,83] Lipase [53,54,81,82] Protease [82] cutinase [84] |
| Thermoplastic polyester PU | Lipases [85,86] Esterases [63,83,85] Pancreatin [87] Polyamidase [15] Proteases [87,88] |
| Thermoplastic polyether PU | Esterase [88,89] Chymotrypsin [88,89,90] Proteases [88,89,90,91] |
| Thermoplastic Polycarbonate PU | Cholesterol esterase [92,93] |
| Thermoplastic poly (ester ether) PU | Chymotrypsin [94] |
| Thermoplastic poly (ester urea) PU | Lipase [95] Cholesterol esterase [96,97,98] |
| Thermoplastic poly (ether urea) PU | Cholesterol esterase [97] Elastase [99] Papain [100] |
| Polyester PU coating | Lipase [101] |
| Polyacryl PU coating | Pancreatin [102] |
| Large Scale Application | Reaction Conditions | Slabstock Recovery Possibility | Environmental Impact | |
|---|---|---|---|---|
| Landfilling | Yes | Moderate | No | High land usage, possibility of fire, Accumulation in environment Can be applied for postconsumer waste |
| Mechanical recycling | Yes | Moderate to extreme | No | Applied only to postproduction waste Some of processes demand high temperature and pressure |
| Chemical recycling | Only glycolysis | Extreme | Yes | Demands high temperatures and pressure Organic solvents, catalysators, and other potentially dangerous chemicals are needed Can be applied for postconsumer waste |
| Energy recovery | Yes | Extreme | No | Toxic fumes release Ashes need to be landfilled Can be applied for postconsumer waste |
| Biological degradation | No | Moderate | Yes | Possibility of toxic compounds release Can be applied for postconsumer waste Can lead to complete mineralization of waste Can be applied on already existing landfills |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. https://doi.org/10.3390/polym12081752
Kemona A, Piotrowska M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers. 2020; 12(8):1752. https://doi.org/10.3390/polym12081752
Chicago/Turabian StyleKemona, Aleksandra, and Małgorzata Piotrowska. 2020. "Polyurethane Recycling and Disposal: Methods and Prospects" Polymers 12, no. 8: 1752. https://doi.org/10.3390/polym12081752