Effect of Toughening with Different Liquid Rubber on Dielectric Relaxation Properties of Epoxy Resin
Abstract
:1. Introduction
2. Materials and Samples Preparation
2.1. Materials
2.2. Sample Preparation
2.3. Performance Measurement
3. Influence of Four Kinds of Rubbers on Glass Transition Temperature
4. Microstructure and Microcrack Development
5. Influence of Four Kinds of Liquid Rubber on Dielectric Properties
5.1. Analysis of DC Resistivity
5.2. Analysis of Relative Permittivity and Dielectric Loss
5.2.1. Relative Permittivity and Dielectric Loss at Different Rubber Contents
5.2.2. Temperature Spectrum and Frequency Spectrum of Samples with Different Liquid Rubber
5.3. Analysis of Relaxation Processes
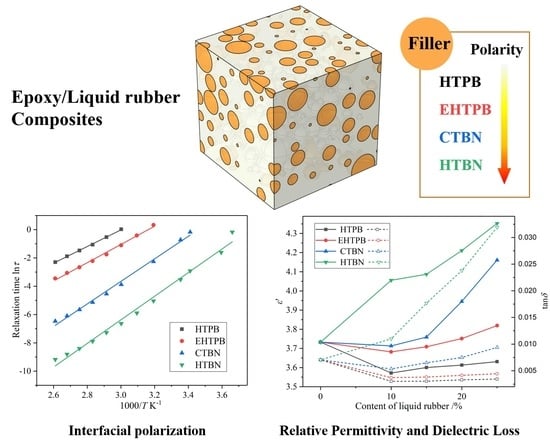
5.3.1. Interfacial Polarization
5.3.2. Relaxation Processes in the Low Temperature Range
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.; Yu, S.; Feng, Y. Progress in and prospects for electrical insulating materials. High Volt. 2016, 1, 122–129. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Guo, Z.; Wang, H.; Zhang, Y.; Hong, R.; Peng, Z. Effects of silane coupling agents on the electrical properties of silica/epoxy nanocomposites. IEEE Int. Conf. Dielectr. (ICD) 2016, 1036–1039. [Google Scholar]
- MacKinnon, A.J.; Jenkins, S.D.; McGrail, P.T.; Pethrick, R.A. A dielectric, mechanical, rheological and electron microscopy study of cure and properties of a thermoplastic-modified epoxy resin. Macromolecules 1992, 25, 3492–3499. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.; Tian, H.; Wang, C.; Guo, Z.; Liu, P.; Peng, Z.; Wang, Q. Synergetic enhancement of mechanical and electrical strength in epoxy/silica nanocomposites via chemically-bonded interface. Compos. Sci. Technol. 2018, 167, 539–546. [Google Scholar] [CrossRef]
- Pan, G. Application of reactive group terminated polybutadiene series liquid rubber in toughened epoxy resins. Chem. Adhes. 2002, 3, 123–126. [Google Scholar]
- Liu, L.; Zhang, H.; Zhang, N.; Weng, L. The preparation and application of CTBN modified epoxy adhesive. Pigm. Resin. Technol. 2015, 44, 358–363. [Google Scholar] [CrossRef]
- Yee, A.; Pearson, R. Toughening mechanisms in elastomer-modified epoxies: 1. Mechanical studies. J. Mater. Sci. 1986, 21, 2462–2474. [Google Scholar] [CrossRef] [Green Version]
- Garima, T.; Deepak, S. Effect of carboxyl-terminated poly (butadiene-co-acrylonitrile) (CTBN) concentration on thermal and mechanical properties of binary blends of diglycidyl ether of bisphenol-A (DGEBA) epoxy resin. Mater. Sci. Eng. A 2007, 443, 262–269. [Google Scholar]
- Zeng, M.; Sun, X.; Xiao, H. Investigation of free volume and the interfacial, and toughening behavior for epoxy resin/rubber composites by positron annihilation. Radiat. Phys. Chem. 2008, 77, 245–251. [Google Scholar]
- Zhou, W.; Cai, J. Mechanical and dielectric properties of epoxy resin modified using reactive liquid rubber (HTPB). J. Appl. Polym. Sci. 2012, 124, 4346–4351. [Google Scholar] [CrossRef]
- Zhou, W.; Cai, J. Mechanical, thermal and electrical proper ties of epoxy modified with a reactive hydroxyl-terminated polystyrene-butadiene liquid rubber. J. Reinf. Plast. Compos. 2013, 32, 1359–1369. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, W.; Kou, Y.; Xu, L.; Wu, H.; Zhao, W. Heat conductive h-BN/CTPB/epoxy with enhanced dielectric properties for potential high-voltage applications. High Volt. 2017, 2, 172–178. [Google Scholar] [CrossRef]
- Kou, Y.; Zhou, W.; Li, B.; Dong, L.; Duan, Y.-E.; Hou, Q.; Liu, X.; Cai, H.; Chen, Q.; Dang, Z.-M. Enhanced mechanical and dielectric properties of an epoxy resin modified with hydroxyl-terminated polybutadiene. Compos. Part A 2018, 114, 97–106. [Google Scholar] [CrossRef]
- Soares, B.G.; Leyva, M.E.; Moreira, V.X.; Barcia, F.L.; Khastgir, D.; Simão, R.A. Morphology and dielectric properties of an epoxy network modified by end-functionalized liquid polybutadiene. J. Polym. Sci. Pol. Phys. 2010, 42, 4053–4062. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, X.; Zhu, R.; Ling, H.; Gu, Y. Thermal and dielectric properties of epoxy/DDS/CTBN adhesive modified by cardanol-based benzoxazine. J. Adhes. Sci. Technol. 2015, 29, 767–777. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Zhang, H.; Wang, H.; Liu, L.; Xu, Z.; Liu, P.; Peng, Z. Influence of addition of hydroxyl-terminated liquid nitrile rubber on dielectric properties and relaxation behavior of epoxy resin. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2258–2269. [Google Scholar] [CrossRef]
- Bian, X.; Tuo, R.; Yang, W.; Zhang, Y.; Xie, Q.; Zha, J.; Lin, J.; He, S. Mechanical, Thermal, and Electrical Properties of BN–Epoxy Composites Modified with Carboxyl-Terminated Butadiene Nitrile Liquid Rubber. Polymers 2019, 11, 1548. [Google Scholar] [CrossRef] [Green Version]
- Kamar, N.; Drzal, L. Micron and Nanostructured Rubber Toughened Epoxy: A Direct Comparison of Mechanical, Thermomechanical and Fracture Properties. Polymer 2016, 92, 114–124. [Google Scholar] [CrossRef]
- Wise, C.; Cook, W.; Goodwin, A. CTBN rubber phase precipitation in model epoxy resins. Polymer 2000, 41, 4625–4633. [Google Scholar] [CrossRef]
- Calabrese, L.; Valenza, A. The effect of a liquid CTBN rubber modifier on the thermo-kinetic parameters of an epoxy resin during a pultrusion process. Compos. Sci. Technol. 2003, 63, 851–860. [Google Scholar] [CrossRef]
- Pascault, J.; Williams, R. Glass transition temperature versus conversion relationships for thermosetting polymers. J. Polym. Sci. Pol. Chem. 2010, 28, 85–95. [Google Scholar] [CrossRef]
- Min, D.; Li, S.; Hirai, N.; Ohki, Y. Dielectric spectroscopic analysis of degradation in ethylene-propylene-diene copolymer. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3620–3630. [Google Scholar] [CrossRef]
- Singha, S.; Thomas, M. Dielectric properties of epoxy nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 12–23. [Google Scholar] [CrossRef]
- Tsangaris, M.; Psarras, G.; Kouloumbi, N. Electric modulus and interfacial polarization in composite polymeric systems. J. Mater. Sci. 1998, 33, 2027–2037. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin, Germany, 2003; pp. 60–63. [Google Scholar]
- Tomer, V.; Polizos, G.; Randall, C.A.; Manias, E. Epoxy-based nanocomposites for electrical energy storage. I: Effects of montmorillonite and barium titanate nanofillers. J. Appl. Phys. 2010, 108, 074116. [Google Scholar] [CrossRef]









| Types of Liquid Rubber (15%) | Apparent Activation Energy Ea/kJ·mol−1 | Determination Coefficient |
|---|---|---|
| HTPB | 48.8 | 0.999 |
| EHTPB | 53.6 | 0.994 |
| CTBN | 66.1 | 0.992 |
| HTBN | 69.3 | 0.988 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Sun, Q.; Lei, K.; Chen, C.; Yao, L.; Peng, Z. Effect of Toughening with Different Liquid Rubber on Dielectric Relaxation Properties of Epoxy Resin. Polymers 2020, 12, 433. https://doi.org/10.3390/polym12020433
Wang C, Sun Q, Lei K, Chen C, Yao L, Peng Z. Effect of Toughening with Different Liquid Rubber on Dielectric Relaxation Properties of Epoxy Resin. Polymers. 2020; 12(2):433. https://doi.org/10.3390/polym12020433
Chicago/Turabian StyleWang, Chuang, Qing Sun, Kang Lei, Chi Chen, Lixiao Yao, and Zongren Peng. 2020. "Effect of Toughening with Different Liquid Rubber on Dielectric Relaxation Properties of Epoxy Resin" Polymers 12, no. 2: 433. https://doi.org/10.3390/polym12020433