Optimization of a New Antioxidant Formulation Using a Simplex Lattice Mixture Design of Apium graveolens L., Coriandrum sativum L., and Petroselinum crispum M. Grown in Northern Morocco
Abstract
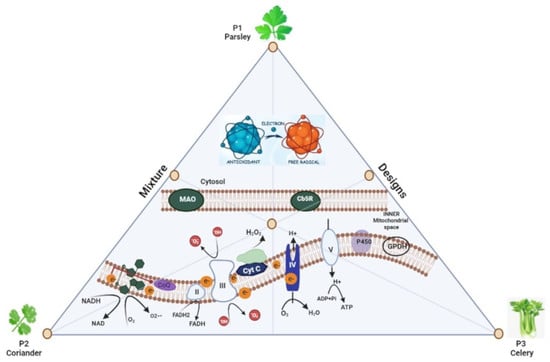
:1. Introduction
2. Results and Discussion
2.1. Screening Study
2.2. Mixture Design of Experiments
2.2.1. Modeling and Analysis of the Statistical Properties of the Mixture Design
2.2.2. Statistical Modeling of the TPC, DPPH, TAC Model
2.3. Diagnostic Plot Analysis
2.3.1. TPC Model
2.3.2. TAC Model
2.3.3. DPPH Model
2.4. Numerical Optimization Using Desirability Function
3. Materials and Methods
3.1. Samples
3.2. Extraction Procedure
3.3. Total Phenolic Content (TPC)
3.4. Evaluation of the Antioxidant Activity
3.4.1. DPPH Free Radical Scavenging Test
3.4.2. Total Antioxidant Capacity (TAC) Test
3.5. Experimental Methodology (Mixture Design of Experiment)
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kara, M.; Assouguem, A.; Fadili, M.E.; Benmessaoud, S.; Alshawwa, S.Z.; Kamaly, O.A.; Saghrouchni, H.; Zerhouni, A.R.; Bahhou, J. Contribution to the Evaluation of Physicochemical Properties, Total Phenolic Content, Antioxidant Potential, and Antimicrobial Activity of Vinegar Commercialized in Morocco. Molecules 2022, 27, 770. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Luo, Q.; Li, T.; Meng, P.-H.; Pu, Y.-T.; Liu, J.-X.; Zhang, J.; Liu, H.; Tan, G.-F.; Xiong, A.-S. Origin, Evolution, Breeding, and Omics of Apiaceae: A Family of Vegetables and Medicinal Plants. Hortic. Res. 2022, 9, uhac076. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regassa, H.; Sourirajan, A.; Kumar, V.; Pandey, S.; Kumar, D.; Dev, K. A Review of Medicinal Plants of the Himalayas with Anti-Proliferative Activity for the Treatment of Various Cancers. Cancers 2022, 14, 3898. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Sauceda, A.E.Q.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Wall-Medrano, A.; de la Rosa, L.A.; González-Aguilar, G.A.; Álvarez-Parrilla, E. Biological Actions of Phenolic Compounds. In Fruit and Vegetable Phytochemicals; Yahia, E.M., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 125–138. ISBN 978-1-119-15804-2. [Google Scholar]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Gligorijevic, N.; Radomirovic, M.; Nedic, O.; Stojadinovic, M.; Khulal, U.; Stanic-Vucinic, D.; Cirkovic Velickovic, T. Molecular Mechanisms of Possible Action of Phenolic Compounds in COVID-19 Protection and Prevention. Int. J. Mol. Sci. 2021, 22, 12385. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an Important Source of Antioxidants and Their Applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Lacroix, M.; Ouattara, B. Combined Industrial Processes with Irradiation to Assure Innocuity and Preservation of Food Products—A Review. Food Res. Int. 2000, 33, 719–724. [Google Scholar] [CrossRef]
- Es-safi, I.; Mechchate, H.; Amaghnouje, A.; Kamaly, O.M.A.; Jawhari, F.Z.; Imtara, H.; Grafov, A.; Bousta, D. The Potential of Parsley Polyphenols and Their Antioxidant Capacity to Help in the Treatment of Depression and Anxiety: An In Vivo Subacute Study. Molecules 2021, 26, 2009. [Google Scholar] [CrossRef]
- Aćimović, M.G. Nutraceutical Potential of Apiaceae. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 1311–1341. ISBN 978-3-319-78029-0. [Google Scholar]
- Majeed, M.; Hussain, A.I.; Chatha, S.A.S.; Khosa, M.K.K.; Kamal, G.M.; Kamal, M.A.; Zhang, X.; Liu, M. Optimization Protocol for the Extraction of Antioxidant Components from Origanum Vulgare Leaves Using Response Surface Methodology. Saudi J. Biol. Sci. 2016, 23, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, V.P.; Aazza, S.; Bertolucci, S.K.V.; Rocha, J.P.M.; Coelho, A.D.; Oliveira, A.J.M.; Mendes, L.C.; Pereira, M.M.A.; Morais, L.C.; Forim, M.R.; et al. Solvent Mixture Optimization in the Extraction of Bioactive Compounds and Antioxidant Activities from Garlic (Allium sativum L.). Molecules 2021, 26, 6026. [Google Scholar] [CrossRef]
- Kooti, W.; Ali-Akbari, S.; Asadi-Samani, M.; Ghadery, H.; Ashtary-Larky, D. A Review on Medicinal Plant of Apium Graveolens. Future Nat. Prod. 2015, 1, 48–59. [Google Scholar]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) Essential Oil: Chemistry and Biological Activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Baratta, M.T.; Dorman, H.J.D.; Deans, S.G.; Biondi, D.M.; Ruberto, G. Chemical Composition, Antimicrobial and Antioxidative Activity of Laurel, Sage, Rosemary, Oregano and Coriander Essential Oils. J. Essent. Oil Res. 1998, 10, 618–627. [Google Scholar] [CrossRef]
- Marthe, F. Petroselinum Crispum (Mill.) Nyman (Parsley). In Medicinal, Aromatic and Stimulant Plants; Novak, J., Blüthner, W.-D., Eds.; Handbook of Plant Breeding; Springer International Publishing: Cham, Switzerland, 2020; Volume 12, pp. 435–466. ISBN 978-3-030-38791-4. [Google Scholar]
- Sousa, R.M.O.F.; Cunha, A.C.; Fernandes-Ferreira, M. The Potential of Apiaceae Species as Sources of Singular Phytochemicals and Plant-Based Pesticides. Phytochemistry 2021, 187, 112714. [Google Scholar] [CrossRef]
- Derouich, M.; Bouhlali, E.D.T.; Bammou, M.; Hmidani, A.; Sellam, K.; Alem, C. Bioactive Compounds and Antioxidant, Antiperoxidative, and Antihemolytic Properties Investigation of Three Apiaceae Species Grown in the Southeast of Morocco. Scientifica 2020, 2020, 3971041. [Google Scholar] [CrossRef]
- Çakır, D.K.; Zannou, O.; Koca, I. Scopoletin Contents and Antioxidant Properties of Some Edible Plants of Black Sea Regions. Discov. Food 2022, 2, 7. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, D.; Liu, X.; Cheng, J.; Wang, X.; Guo, D.; Zou, J.; Yang, H. Phenolic Profile and Antioxidant Activity of Longan Pulp of Different Cultivars from South China. LWT 2022, 165, 113698. [Google Scholar] [CrossRef]
- Ouedrhiri, W.; Mechchate, H.; Moja, S.; Baudino, S.; Saleh, A.; Al Kamaly, O.M.; Grafov, A.; Greche, H. Optimized Antibacterial Effects in a Designed Mixture of Essential Oils of Myrtus communis, Artemisia herba-alba and Thymus serpyllum for Wide Range of Applications. Foods 2022, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern Extraction Methods for Preparation of Bioactive Plant Extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Kraemer, K.; Harwardt, A.; Bronneberg, R.; Marquardt, W. Separation of Butanol from Acetone–Butanol–Ethanol Fermentation by a Hybrid Extraction–Distillation Process. Comput. Chem. Eng. 2011, 35, 949–963. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Salih, A.M.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M.; Khan, S.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alfagham, A.; Alkahtani, J. Optimization Method for Phenolic Compounds Extraction from Medicinal Plant (Juniperus procera) and Phytochemicals Screening. Molecules 2021, 26, 7454. [Google Scholar] [CrossRef]
- Aazza, S. Application of Multivariate Optimization for Phenolic Compounds and Antioxidants Extraction from Moroccan Cannabis sativa Waste. J. Chem. 2021, 2021, 9738656. [Google Scholar] [CrossRef]
- Achat, S. Polyphénols de l’Alimentation: Extraction, Pouvoir Antioxydant et Interactions avec des Ions Métalliques. Ph.D. Thesis, Université d’Avignon; Université Abderrahmane Mira, Bejaïa, Algeria; p. 261.
- Youmbai, A.; Mehellou, Z.; Boual, Z.; Gardarin, C.; Pierre, G.; Delattre, D.; Michaud, P.; Ould El-Hadj, M.P. Characterization and Biological Activities of a Polysaccharidic Extract from Ferula communis L. (Apiaceae) Harvested in Sahara. Phytothérapie 2021, 20, 205–213. [Google Scholar] [CrossRef]
- Tunçtürk, M.; Özgökçe, F. Chemical Composition of Some Apiaceae Plants Commonly Used in Herby Cheese in Eastern Anatolia. Turk. J. Agric. For. 2015, 39, 55–62. [Google Scholar] [CrossRef]
- Shamsiev, A.; Park, J.; Olawuyi, I.F.; Odey, G.; Lee, W. Optimization of Ultrasonic-Assisted Extraction of Polyphenols and Antioxidants from Cumin (Cuminum cyminum L.). Korean J. Food Preserv. 2021, 28, 510–521. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Abdul Manap, M.; Khatib, A.; Abdull Razis, A. Comparative Analysis of Chemical Composition, Antioxidant Activity and Quantitative Characterization of Some Phenolic Compounds in Selected Herbs and Spices in Different Solvent Extraction Systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [Green Version]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Ez zoubi, Y.; Fadil, M.; Bousta, D.; El Ouali Lalami, A.; Lachkar, M.; Farah, A. Ultrasound-Assisted Extraction of Phenolic Compounds from Moroccan Lavandula stoechas L.: Optimization Using Response Surface Methodology. J. Chem. 2021, 2021, 8830902. [Google Scholar] [CrossRef]
- Mechchate, H.; Ouedrhiri, W.; Es-safi, I.; Amaghnouje, A.; Jawhari, F.z.; Bousta, D. Optimization of a New Antihyperglycemic Formulation Using a Mixture of Linum usitatissimum L., Coriandrum sativum L., and Olea europaea Var. sylvestris Flavonoids: A Mixture Design Approach. Biologics 2021, 1, 154–163. [Google Scholar] [CrossRef]
- Derouich, M.; Bouhlali, E.D.T.; Hmidani, A.; Bammou, M.; Bourkhis, B.; Sellam, K.; Alem, C. Assessment of Total Polyphenols, Flavonoids and Anti-Inflammatory Potential of Three Apiaceae Species Grown in the Southeast of Morocco. Sci. Afr. 2020, 9, e00507. [Google Scholar] [CrossRef]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic Properties and Drug Interactions of Apigenin, a Natural Flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Phenolic Composition and Antioxidant Activities of 11 Celery Cultivars. J. Food Sci. 2010, 75, C9–C13. [Google Scholar] [CrossRef]
- Majda, E.; Bouchra, L.; Faiçal El, O.; Abdelhak, B.; Noureddine, E. Application of Response Surface Methodology to Optimize the Extraction of Essential Oil from Rosmarinus officinalis Using Microwave-Assisted Hydrodistillation. J. Appl. Pharm. Sci. 2020, 11, 129–136. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-safi, I.; Amaghnouje, A.; Boukhira, S.; Alotaibi, A.A.; Al-zharani, M.; Nasr, F.A.; Noman, O.M.; Conte, R.; Amal, E.H.E.Y.; et al. Antioxidant, Anti-Inflammatory and Antidiabetic Proprieties of LC-MS/MS Identified Polyphenols from Coriander Seeds. Molecules 2021, 26, 487. [Google Scholar] [CrossRef]
- Aquino, L.P.; Borges, S.V.; Queiroz, F.; Antoniassi, R.; Cirillo, M.A. Extraction of Oil from Pequi Fruit (Caryocar brasiliense, Camb.) Using Several Solvents and Their Mixtures. Grasas Aceites 2011, 62, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Dehghani, M.H.; Hassani, A.H.; Karri, R.R.; Younesi, B.; Shayeghi, M.; Salari, M.; Zarei, A.; Yousefi, M.; Heidarinejad, Z. Process Optimization and Enhancement of Pesticide Adsorption by Porous Adsorbents by Regression Analysis and Parametric Modelling. Sci. Rep. 2021, 11, 11719. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural Polyphenols (Vegetable Tannins) as Drugs: Possible Modes of Action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.; Simonetti, P.; Mauri, P. Antioxidant Activity of Selected Medicinal Plants. J. Agric. Food Chem. 1998, 46, 4487–4490. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total Antioxidant Capacities of Raw and Cooked Meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ranneh, Y.; Abu Bakar, M.F.; Ismail, N.A.; Kormin, F.; Mohamed, M.; Md Akim, A.; Isha, A. Anti-Aging and Antioxidant of Four Traditional Malaysian Plants Using Simplex Centroid Mixture Design Approach. Saudi J. Biol. Sci. 2021, 28, 6711–6720. [Google Scholar] [CrossRef]
- de Menezes, B.B.; Frescura, L.M.; Duarte, R.; Villetti, M.A.; da Rosa, M.B. A Critical Examination of the DPPH Method: Mistakes and Inconsistencies in Stoichiometry and IC50 Determination by UV–Vis Spectroscopy. Anal. Chim. Acta 2021, 1157, 338398. [Google Scholar] [CrossRef]
- El Ghouizi, A.; El Menyiy, N.; Falcão, S.I.; Vilas-Boas, M.; Lyoussi, B. Chemical Composition, Antioxidant Activity, and Diuretic Effect of Moroccan Fresh Bee Pollen in Rats. Vet. World 2020, 13, 1251–1261. [Google Scholar] [CrossRef]
- El Ouadrhiri, F.; Elyemni, M.; Lahkimi, A.; Lhassani, A.; Chaouch, M.; Taleb, M. Mesoporous Carbon from Optimized Date Stone Hydrochar by Catalytic Hydrothermal Carbonization Using Response Surface Methodology: Application to Dyes Adsorption. Int. J. Chem. Eng. 2021, 2021, 5555406. [Google Scholar] [CrossRef]
- Hao, H.; Zhu, X.; Zhang, X.; Zhang, C. R-Optimal Design of the Second-Order Scheffé Mixture Model. Stat. Probabil. Lett. 2021, 173, 109069. [Google Scholar] [CrossRef]
- Aboulghazi, A.; Bakour, M.; Fadil, M.; Lyoussi, B. Simultaneous Optimization of Extraction Yield, Phenolic Compounds and Antioxidant Activity of Moroccan Propolis Extracts: Improvement of Ultrasound-Assisted Technique Using Response Surface Methodology. Processes 2022, 10, 297. [Google Scholar] [CrossRef]
- Nunes Filho, R.C.; Galvan, D.; Effting, L.; Terhaag, M.M.; Yamashita, F.; de Toledo Benassi, M.; Spinosa, W.A. Effects of Adding Spices with Antioxidants Compounds in Red Ale Style Craft Beer: A Simplex-Centroid Mixture Design Approach. Food Chem. 2021, 365, 130478. [Google Scholar] [CrossRef]






| Test Runs | Independent Variable | Measured Response | |||||
|---|---|---|---|---|---|---|---|
| Component 1 | Component 2 | Component 3 | Response 1 | Response 2 | Response 3 | ||
| P1 % | P2 % | P3 % | Yield (%) | DPPH (Activity %) | TAC (Mg AscE/g) DW | TPC (Mg GAE/g) DW | |
| 1 | 0.1 | 0.33 | 0.57 | 11.68% | 16.67 ± 1.98 defghij | 23.33 ± 0.40 i | 5.65 ± 0.05 bcdefj |
| 2 | 0.57 | 0.33 | 0.1 | 20.55% | 55.85 ± 0.21 a | 73.31 ± 0.11 b | 21.95 ± 0.03 a |
| 3 | 0.1 | 0.1 | 0.8 | 20.99% | 23.88 ± 0.32 de | 12.25 ± 0.06 klm | 7.79 ± 0.05 bcd |
| 4 | 0.1 | 0.1 | 0.8 | 20.87% | 21.54 ± 0.44 defg | 13.15 ± 0.06 klm | 7.66 ± 0.08 bcde |
| 5 | 0.33 | 0.1 | 0.57 | 21.64% | 26.26 ± 0.93 d | 27.92 ± 0.06 g | 9.15 ± 0.08 bcd |
| 6 | 0.57 | 0.1 | 0.33 | 23.24% | 13.16 ± 0.14 k | 13.90 ± 0.09 kl | 7.55 ± 0.08 bcde |
| 7 | 0.33 | 0.57 | 0.1 | 20.34% | 22.63 ± 0.11 def | 25.76 ± 0.29 h | 8.08 ± 0.08 bcd |
| 8 | 0.33 | 0.33 | 0.33 | 19.2% | 25.32 ± 0.55 d | 62.51 ± 0.34 d | 10.82 ± 0.05 b |
| 9 | 0.33 | 0.57 | 0.1 | 19.99% | 20.39 ± 0.41 defg | 28.12 ± 0.29 g | 8.37 ± 0.05 bcd |
| 10 | 0.8 | 0.1 | 0.1 | 21.68% | 18.98 ± 0.48 defgh | 12.58 ± 0.23 klm | 7.24 ± 0.08 bcde |
| 11 | 0.22 | 0.22 | 0.57 | 20.45% | 20.85 ± 0.34 defg | 22.31 ± 0.11 ij | 8.92 ± 0.08 bcd |
| 12 | 0.1 | 0.8 | 0.1 | 19.13% | 41.07 ± 0.37 b | 69.08 ± 0.46 c | 11.51 ± 0.05 b |
| 13 | 0.8 | 0.1 | 0.1 | 21.88% | 18.33 ± 0.35 defghi | 14.28 ± 0.06 k | 7.49 ± 0.05 bcde |
| 14 | 0.33 | 0.33 | 0.33 | 20.73% | 26.41 ± 0.74 d | 63.28 ± 0.29 d | 10.53 ± 0.03 bc |
| 15 | 0.1 | 0.57 | 0.33 | 19.29% | 39.26 ± 0.74 c | 21.63 ± 0.46 ij | 6.28 ± 0.03 bcdef |
| 16 | 0.57 | 0.1 | 0.33 | 19.87% | 13.65 ± 0.53 k | 14.83 ± 0.37 k | 7.12 ± 0.08 bcdef |
| 17 | 0.33 | 0.1 | 0.57 | 20.56% | 20.60 ± 0.62 defg | 30.34 ± 0.03 f | 9.17 ± 0.05 bcd |
| 18 | 0.1 | 0.57 | 0.33 | 19.41% | 39.12 ± 0.45 c | 21.87 ± 0.40 ij | 6.32 ± 0.08 bcdef |
| 19 | 0.22 | 0.57 | 0.22 | 18.49% | 23.54 ± 0.89 def | 31.15 ± 0.03 f | 8.89 ± 0.08 bcd |
| 20 | 0.1 | 0.8 | 0.1 | 18.61% | 42.38 ± 0.22 b | 69.29 ± 0.06 c | 11.95 ± 0.03 b |
| 21 | 0.1 | 0.33 | 0.57 | 20.49% | 16.79 ± 0.60 defghij | 22.48 ± 0.57 ij | 5.57 ± 0.05 bcdefg |
| 22 | 0.57 | 0.33 | 0.1 | 20.22% | 56.21 ± 0.10 a | 75.82 ± 0.34 a | 21.98 ± 0.03 a |
| 23 | 0.57 | 0.22 | 0.22 | 20.45% | 17.61 ± 0.91 defghi | 37.25 ± 0.57 e | 5.74 ± 0.08 bcdef |
| Variables | TPC | TAC | DPPH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-Value | F-Value | Coefficient | p-Value | F-Value | Coefficient | p-Value | F-Value | |
| Model | 12,974.50 | <0.0001 * | 54.21 | 9868.98 | <0.0001 * | 14.93 | 3149.79 | <0.0001 * | 18.68 |
| Linear Mixture | 2648.64 | <0.0001 * | 49.80 | 3307.56 | <0.0001 * | 22.52 | 939.76 | <0.0001 * | 25.08 |
| AB | 585.50 | 0.0004 * | 22.02 | 189.47 | 0.1322 ** | 2.58 | 125.06 | 0.0227 * | 6.68 |
| AC | 57.85 | 0.1641 ** | 2.18 | 98.83 | 0.2669 ** | 1.35 | 14.64 | 0.3927 ** | 0.7816 |
| BC | 2718.60 | <0.0001 * | 102.22 | 673.49 | 0.0097 * | 9.17 | 24.76 | 0.2710 ** | 1.32 |
| ABC | 850.19 | <0.0001 * | 31.97 | 885.63 | 0.0041 * | 12.06 | 47.52 | 0.1353 ** | 2.54 |
| AB(A-B) | 5072.34 | <0.0001 * | 190.73 | 3819.72 | <0.0001 * | 52.01 | 1594.21 | <0.0001 * | 85.09 |
| AC(A-C) | 490.80 | 0.0009 * | 18.45 | 265.75 | 0.0795 ** | 3.62 | 212.18 | 0.0051 * | 11.33 |
| BC(B-C) | 6.31 | 0.6343 ** | 0.2373 | 191.42 | 0.1304 ** | 2.61 | 251.02 | 0.0029 ** | 13.40 |
| Residual | 345.73 | 954.73 | 243.56 | ||||||
| Lack of Fit | 311.26 | <0.0001 * | 30.10 | 942.78 | <0.0001 * | 262.96 | 220.43 | <0.0001 * | 31.77 |
| R2 | 0.97 | 0.91 | 0.93 | ||||||
| R2adjusted | 0.95 | 0.85 | 0.88 | ||||||
| R2predicted | 0.93 | 0.79 | 0.82 | ||||||
| Response | The Equations |
|---|---|
| TPC | TPC= −20.86 × A + 286.63 × B + 74.07 × C − 72.57 × AB + 4.77 × AC − 611.21 × BC + 1825.67 × ABC + 1335.16 × AB(A-B) − 415.32 × AC(A-C) − 47.1 × BC(B-C) |
| TAC | TAC= −70.38 × A + 236.04 × B − 19.87 × C − 153.96 × AB + 19.65 × AC − 439.2 × BC + 1 863.33 × ABC + 1158.63 × AB(A-B) − 305.61 × AC(A-C) − 259.37 × BC(B-C) |
| DPPH | DPPH = 0.96 × A − 0.34 × B + 0.67 × C − 1.07 × AB − 0.7 × AC + 0.27 × BC + 2.61 × ABC − 7.24 × AB(A-B) + 3.01 × AC(A-C) − 0.19 × BC(B-C) |
| Optimal Condition: XP1 = 0.611, XP2 = 0.289, XP2 = 0.100 Desirability: 0.966 | |||
|---|---|---|---|
| Predicted | Experimental | Error | |
| TPC (i) | 21.22 | 21.93 | 0.71 (1.1%) * |
| TAC (ii) | 72.37 | 72.74 | 0.37 (0.5%) * |
| DPPH (iii) | 55.05 | 56.00 | 0.95 (1.2%) * |
| Component | Name | Units | Type | Coded Low | Coded High |
|---|---|---|---|---|---|
| A | P1 | % | Mixture | +0 ↔ 0.1 | +1 ↔ 0.8 |
| B | P2 | % | Mixture | +0 ↔ 0.1 | +1 ↔ 0.8 |
| C | P3 | % | Mixture | +0 ↔ 0.1 | +1 ↔ 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouioura, G.; Tourabi, M.; El Ghouizi, A.; Kara, M.; Assouguem, A.; Saleh, A.; Kamaly, O.A.; El Ouadrhiri, F.; Lyoussi, B.; Derwich, E.H. Optimization of a New Antioxidant Formulation Using a Simplex Lattice Mixture Design of Apium graveolens L., Coriandrum sativum L., and Petroselinum crispum M. Grown in Northern Morocco. Plants 2023, 12, 1175. https://doi.org/10.3390/plants12051175
Nouioura G, Tourabi M, El Ghouizi A, Kara M, Assouguem A, Saleh A, Kamaly OA, El Ouadrhiri F, Lyoussi B, Derwich EH. Optimization of a New Antioxidant Formulation Using a Simplex Lattice Mixture Design of Apium graveolens L., Coriandrum sativum L., and Petroselinum crispum M. Grown in Northern Morocco. Plants. 2023; 12(5):1175. https://doi.org/10.3390/plants12051175
Chicago/Turabian StyleNouioura, Ghizlane, Meryem Tourabi, Asmae El Ghouizi, Mohammed Kara, Amine Assouguem, Asmaa Saleh, Omkulthom Al Kamaly, Faiçal El Ouadrhiri, Badiaa Lyoussi, and El Houssine Derwich. 2023. "Optimization of a New Antioxidant Formulation Using a Simplex Lattice Mixture Design of Apium graveolens L., Coriandrum sativum L., and Petroselinum crispum M. Grown in Northern Morocco" Plants 12, no. 5: 1175. https://doi.org/10.3390/plants12051175