Investigation of Combined Cyclodextrin and Hydrogel Formulation for Ocular Delivery of Dexamethasone Acetate by Means of Experimental Designs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Quantitative Determinations
2.2.2. Phase Solubility Diagrams
2.2.3. Chromatographic Determination of the Association Constants
2.2.4. Thermodynamic Parameters for the DXMa/Cyclodextrin Complexes
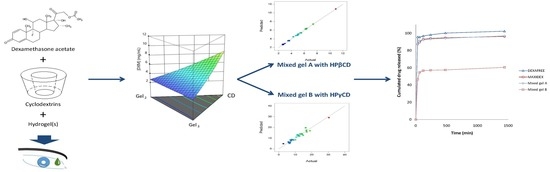
2.2.5. Experimental Designs and Data Analysis
2.2.6. Rheological Characterization
2.2.7. In Vitro DXMa Release Profiles
3. Results and Discussion
3.1. Solubility Determinations of Dexamethasone Acetate
3.2. Chromatographic Determination of the Association Constants Between Dexamethasone Acetate and HPβCD or HPγCD
3.3. Thermodynamic Parameters for the DXMa/Cyclodextrin Complexes
3.4. Special Cubic Mixture Designs
3.5. Rheological Characterization
3.6. In Vitro DXM Release Studies
4. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Acharya, N.R.; Tham, V.M.; Esterberg, E.; Borkar, D.S.; Parker, J.V.; Vinoya, A.C.; Uchida, A. Incidence and prevalence of uveitis: Results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. 2013, 131, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Kadam, R.S.; Lee, V.H.L. Recent advances in ophthalmic drug delivery. Ther. Deliv. 2010, 1, 435–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gause, S.; Hsu, K.-H.; Shafor, C.; Dixon, P.; Powell, K.C.; Chauhan, A. Mechanistic modeling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses. Adv. Colloid Interface Sci. 2016, 233, 139–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef] [PubMed]
- Edsman, K.; Hägerström, H. Pharmaceutical applications of mucoadhesion for the non-oral routes. J. Pharm. Pharmacol. 2005, 57, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesavan, K.; Kant, S.; Singh, P.N.; Pandit, J.K. Effect of hydroxypropyl-β-cyclodextrin on the ocular bioavailability of dexamethasone from a pH-induced mucoadhesive hydrogel. Curr. Eye Res. 2011, 36, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Saari, K.M.; Nelimarkka, L.; Ahola, V.; Loftsson, T.; Stefánsson, E. Comparison of topical 0.7% dexamethasone-cyclodextrin with 0.1% dexamethasone sodium phosphate for postcataract inflammation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Ohira, A.; Hara, K.; Jóhannesson, G.; Tanito, M.; Ásgrímsdóttir, G.M.; Lund, S.H.; Loftsson, T.; Stefánsson, E. Topical dexamethasone γ-cyclodextrin nanoparticle eye drops increase visual acuity and decrease macular thickness in diabetic macular oedema. Act. Ophthalmol. 2015, 93, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Stahn, C.; Löwenberg, M.; Hommes, D.W.; Buttgereit, F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol. Cell. Endocrinol. 2007, 275, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koç, F.E.; Senel, M. Solubility enhancement of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) using polypolypropylene oxide core PAMAM dendrimers. Int. J. Pharm. 2013, 451, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Moya-Ortega, M.D.; Messner, M.; Jansook, P.; Nielsen, T.T.; Wintgens, V.; Larsen, K.L.; Amiel, C.; Sigurdsson, H.H.; Loftsson, T. Drug loading in cyclodextrin polymers: Dexamethasone model drug. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 377–382. [Google Scholar] [CrossRef]
- Usayapant, A.; Karara, A.H.; Narurkar, M.M. Effect of 2-hydroxypropyl-beta-cyclodextrin on the ocular absorption of dexamethasone and dexamethasone acetate. Pharm. Res. 1991, 8, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, H.M.; Kupferman, A.; Stewart, R.H.; Kimbrough, R.L. Evaluation of dexamethasone acetate as a topical ophthalmic formulation. Am. J. Ophthalmol. 1978, 86, 418–423. [Google Scholar] [CrossRef]
- Göktürk, S.; Çalışkan, E.; Talman, R.Y.; Var, U. A Study on Solubilization of Poorly Soluble Drugs by Cyclodextrins and Micelles: Complexation and Binding Characteristics of Sulfamethoxazole and Trimethoprim. Sci. World J. 2012, 2012, 718791. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C.C.; Mainardes, R.M.; Gremião, M.P.D. Development and validation of HPLC method for analysis of dexamethasone acetate in microemulsions. Braz. J. Pharm. Sci. 2009, 45, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Connors, K.A. Phase-solubility techniques. In Advanced in Analytical Chemistry and Instrumentation; Wiley-Interscience: New York City, NY, USA, 1965; pp. 117–212. [Google Scholar]
- Jansook, P.; Loftsson, T. gammaCD/HPgammaCD: synergistic solubilization. Int. J. Pharm. 2008, 363, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Ravelet, C.; Geze, A.; Villet, A.; Grosset, C.; Ravel, A.; Wouessidjewe, D.; Peyrin, E. Chromatographic determination of the association constants between nimesulide and native and modified beta-cyclodextrins. J. Pharm. Biomed. Anal. 2002, 29, 425–430. [Google Scholar] [CrossRef]
- Peyrin, E.; Guillaume, Y.C.; Grosset, C.; Villet, A.; Ravel, A.; Alary, J. Sucrose effect on reversed-phase liquid chromatography solute retention. Anal. Chim. Acta 2001, 428, 83–88. [Google Scholar] [CrossRef]
- Chédru-Legros, V.; Fines-Guyon, M.; Chérel, A.; Perdriel, A.; Albessard, F.; Debruyne, D.; Mouriaux, F. Fortified antibiotic (vancomycin, amikacin and ceftazidime) eye drop stability assessment at −20 degrees C. J. Fr. Ophtalmol. 2007, 30, 807–813. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building; Wiley-Probability and Mathematical Statistics: New York City, NY, USA, 1978; ISBN 978-0-471-09315-2. [Google Scholar]
- Yin, Y.; Carter, C.W. Incomplete factorial and response surface methods in experimental design: Yield optimization of tRNA (Trp) from in vitro T7 RNA polymerase transcription. Nucleic Acids Res. 1996, 24, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Bakhary, N.; Abidin, A.R.Z. Response surface methodology for damage detection using frequency and mode shape. Measurement 2018, 115, 258–268. [Google Scholar] [CrossRef]
- Vianna, R.F.; Bentley, M.V.L.; Ribeiro, G.; Carvalho, F.S.; Neto, A.F.; de Oliveira, D.C.; Collett, J.H. Formation of cyclodextrin inclusion complexes with corticosteroids: their characterization and stability. Int. J. Pharm. 1998, 167, 205–213. [Google Scholar] [CrossRef]
- Djedaïni, F.; Perly, B. Nuclear Magnetic Resonance Investigation of the Stoichiometrics in β-Cyclodextrin: Steroid Inclusion Complexes. J. Pharm. Sci. 1991, 80, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Flood, K.G.; Reynolds, E.R.; Snow, N.H. Characterization of inclusion complexes of betamethasone-related steroids with cyclodextrins using high-performance liquid chromatography. J. Chromatogr. A 2000, 903, 49–65. [Google Scholar] [CrossRef]
- Stefánsson, E.; Loftsson, T. Cyclodextrins in Eye Drop Formulations. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 23–27. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes in Pascal: The Art of Scientific Computing; Cambridge University Press: Cambridge, UK, 1989; ISBN 978-0-521-37516-0. [Google Scholar]
- Bothner, H.; Waaler, T.; Wik, O. Rheological Characterization of Tear Substitutes. Drug Dev. Ind. Pharm. 1990, 16, 755–768. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Abd Hamid, S.B. Investigation of optimal conditions for production of highly crystalline nanocellulose with increased yield via novel Cr(III)-catalyzed hydrolysis: Response surface methodology. Carbohydr. Polym. 2017, 178, 57–68. [Google Scholar] [CrossRef] [PubMed]


 ) or HPγCD (
) or HPγCD (  ). Each data point represents a mean (n = 3), with SD smaller than the symbol size.
). Each data point represents a mean (n = 3), with SD smaller than the symbol size.
 ) or HPγCD (
) or HPγCD (  ). Each data point represents a mean (n = 3), with SD smaller than the symbol size.
). Each data point represents a mean (n = 3), with SD smaller than the symbol size.
 ) or [HPγCD] (
) or [HPγCD] (  ) (assuming 1:1 stoichiometry) for dexamethasone acetate at a column temperature equal to 40 °C. Stationary phase: phenyl silica gel; mobile phase: mixture methanol: water (70:30 v/v).
) (assuming 1:1 stoichiometry) for dexamethasone acetate at a column temperature equal to 40 °C. Stationary phase: phenyl silica gel; mobile phase: mixture methanol: water (70:30 v/v).
 ) or [HPγCD] (
) or [HPγCD] (  ) (assuming 1:1 stoichiometry) for dexamethasone acetate at a column temperature equal to 40 °C. Stationary phase: phenyl silica gel; mobile phase: mixture methanol: water (70:30 v/v).
) (assuming 1:1 stoichiometry) for dexamethasone acetate at a column temperature equal to 40 °C. Stationary phase: phenyl silica gel; mobile phase: mixture methanol: water (70:30 v/v).
 ) or DXMa/HPγCD (
) or DXMa/HPγCD (  ) associations.
) associations.
 ) or HPγCD (
) or HPγCD (  ).
).

| Drugs | Retention Time | Calibration Curve | Correlation Coefficient | Intra-Day Variability (CV%) | Inter-Day Variability (CV%) |
|---|---|---|---|---|---|
| DXM | 4.8 | y = (3 × 107)x + 27.867 | 0.999 | <1% | <3% |
| DXM sodium phosphate | 3.8 | y = (5 × 106)x – 312.7 | 0.999 | <1% | <2% |
| DXMa | 6.3 | y = (3 × 107)x + 39.464 | 0.999 | <1% | <3% |
| Component | Low Level (%) | High Level (%) | ||
|---|---|---|---|---|
| Experimental Design 1 | CELLUVISC®-Gel1 | 0 | 70 | |
| GEL-LARMES®-Gel2 | 0 | 70 | ||
| VISMED®-Gel3 | 0 | 70 | ||
| HPβCD 600 mg/mL with DXMa 10 mg/mL | 30 | 100 | ||
| Experimental Design 2 | CELLUVISC®-Gel1 | 0 | 70 | |
| GEL-LARMES®-Gel2 | 0 | 70 | ||
| VISMED®-Gel3 | 0 | 70 | ||
| HPγCD 600 mg/mL with DXMa 30 mg/mL | 30 | 100 | ||
| CD Type | Slope | Correlation Coefficient | K1:1 (M−1) | CE |
|---|---|---|---|---|
| HPβCD | 0.066 | 0.995 | 1462 | 0.071 |
| HPγCD | 0.206 | 0.999 | 5368 | 0.259 |
| Method | Chromatographic Experiments | Phase Solubility Studies | UV Spectroscopy | ||||
|---|---|---|---|---|---|---|---|
| Reference | Present study | Present study | [12] | [12] | |||
| Solution | methanol:water (70:30) | water | water 0.1 M citrate buffer (pH 6.0) | water 0.1 M citrate buffer (pH 6.0) | |||
| Temperature (°C) | 25 | 30 | 35 | 40 | 25 | 25 | 25 |
| HPβCD | 1807 | 1421 | 1234 | 1020 | 1462 | 2240 | 2445 |
| HPγCD | 2541 | 2195 | 1883 | 1787 | 5368 | - | - |
| DXMa/CD Complexes | ΔH° | ΔS° | ΔG° (kJ/mol) | ||
|---|---|---|---|---|---|
| kJ/mol | Contribution to ΔG° | J/mol K | Contribution to ΔG° | ||
| DXMa/HPβCD | −20.3 | 54% | +57.1 | 46% | −3.3 |
| DXMa/HPγCD | −30.7 | 67% | +50.1 | 33% | −15.7 |
| Final Equation in Terms of Actual Components | Model Evaluation | Predicted vs. Actual Plot | ||
|---|---|---|---|---|
| HPβCD | Osmolality | Osmolality(mOsm/Kg) = +342.68 × Gel1 +369.84 × Gel2 +242.96 × Gel3 +765.94 × [HPβCD] −504.45 × Gel1 × [HPβCD] −452.83 × Gel2 × [HPβCD] −757.14 × Gel3 × [HPβCD] | R2 = 0.9900R2adj = 0.9872 R2pred = 0.9849 Adeq Prec = 98.87 BIC = 232.56 AICc = 228.17 |  |
| [DXMa] | [DXMa] (mg/mL) = (+1.47×10−3) × Gel1 +0.50 × Gel2 +1.16 × Gel3 +10.90 × [HPβCD] −2.93 × Gel1 × [HPβCD] −6.01 × Gel2 × Gel3 −4.29 × Gel2 × [HPβCD] −5.49 × Gel3 × [HPβCD] +17.45 × Gel2 × Gel3 × [HPβCD] | R2 = 0.9980 R2adj = 0.9972 R2pred = 0.9932 Adeq Prec = 155.01 BIC = −37.76 AICc = −41.50 |  | |
| HPγCD | Osmolality | Osmolality (mOsm/Kg) = +768.79 × Gel1 +57.80 × Gel2 +329.10 × Gel3 +789.73 × [HPγCD] −1367.82 × Gel1 × Gel2 −1986.36 × Gel1 × [HPγCD] −140.20 × Gel2 × [HPγCD] −1053.47 × Gel3 × [HPγCD] +5990.04 × Gel1 × Gel2 × [HPγCD] | R2 = 0.9403 R2adj = 0.9165 R2pred = 0.8517 AdeqPrec = 31.45 BIC = 297.68 AICc = 293.94 |  |
| [DXMa] | [DXMa] (mg/mL) = +4.36 × Gel1 −5.70 × Gel2 −4.60 × Gel3 +29.10 × [HPγCD] −20.34 × Gel1 × [HPγCD] | R2 = 0.9450 R2adj = 0.9357 R2pred = 0.9055 AdeqPrec = 42.94 BIC = 108.39 AICc = 104.58 |  |
| CD Type | Gel1 CELLUVISC® (%) | Gel2 GEL-LARMES® (%) | Gel3 VISMED® (%) | CD (%) | Actual Osmolality | Predicted Osmolality | Actual[DXMa] (mg/mL) | Predicted[DXMa] (mg/mL) |
|---|---|---|---|---|---|---|---|---|
| HPβCD | 0.000 | 0.000 | 0.300 | 0.700 | 429 | 450.045 | 6.973 | 6.826 |
| HPβCD | 0.000 | 0.145 | 0.215 | 0.640 | 450 | 449.858 | 6.319 | 6.305 |
| HPβCD | 0.454 | 0.000 | 0.000 | 0.546 | 435 | 448.735 | 4.651 | 5.226 |
| HPγCD | 0.089 | 0.089 | 0.098 | 0.724 | 519 | 489.326 | 17.153 | 19.188 |
| HPγCD | 0.000 | 0.425 | 0.000 | 0.575 | 447 | 444.396 | 12.813 | 14.310 |
| HPγCD | 0.244 | 0.201 | 0.000 | 0.555 | 436 | 448.831 | 13.492 | 13.314 |
| Components | Quantity (g) | |
|---|---|---|
| Optimized mixed gel A | VISMED®-Gel3 | 0.300 |
| HPβCD 600 mg/mL with DXMa | 0.700 | |
| Optimized mixed Gel A contains 7 mg/g of DXMa and an osmolality of 449 mOsm/kg | ||
| Optimized mixed gel B | CELLUVISC®-Gel1 | 0.151 |
| VISMED®-Gel3 | 0.085 | |
| HPγCD 600 mg/mL with DXMa | 0.764 | |
| Optimized mixed gel B contains 20 mg/g of DXMa and an osmolality of 425 mOsm/kg | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazet, R.; Choisnard, L.; Levilly, D.; Wouessidjewe, D.; Gèze, A. Investigation of Combined Cyclodextrin and Hydrogel Formulation for Ocular Delivery of Dexamethasone Acetate by Means of Experimental Designs. Pharmaceutics 2018, 10, 249. https://doi.org/10.3390/pharmaceutics10040249
Mazet R, Choisnard L, Levilly D, Wouessidjewe D, Gèze A. Investigation of Combined Cyclodextrin and Hydrogel Formulation for Ocular Delivery of Dexamethasone Acetate by Means of Experimental Designs. Pharmaceutics. 2018; 10(4):249. https://doi.org/10.3390/pharmaceutics10040249
Chicago/Turabian StyleMazet, Roseline, Luc Choisnard, Delphine Levilly, Denis Wouessidjewe, and Annabelle Gèze. 2018. "Investigation of Combined Cyclodextrin and Hydrogel Formulation for Ocular Delivery of Dexamethasone Acetate by Means of Experimental Designs" Pharmaceutics 10, no. 4: 249. https://doi.org/10.3390/pharmaceutics10040249