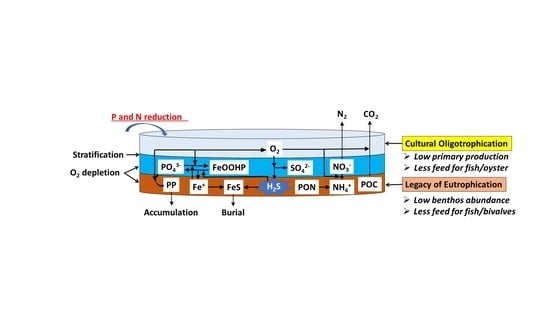
A Conflict between the Legacy of Eutrophication and Cultural Oligotrophication in Hiroshima Bay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Hiroshima Bay
2.2. Field Observations
2.3. Chemical Analyses
2.4. Estimation of the Sediment P Content
3. Results
3.1. Sediment C, N, S, and P Contents
3.2. Subsidiary Parameters Relating to C, N, S and P Cycles
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asmala, E.; Carstensen, J.; Conley, D.; Slomp, C.P.; Stadmark, J.; Voss, M. Efficiency of the coastal filter: Nitrogen and phosphorous removal in the Baltic Sea. Limnol. Oceanogr. 2017, 62, S222–S238. [Google Scholar] [CrossRef]
- Prastka, K.E.; Malcolm, S.J. Particulate phosphorus in the Humber estuary. Neth. J. Aquat. Ecol. 1994, 28, 397–403. [Google Scholar] [CrossRef]
- Bowden, K.F.; Din, S.H.S.E. Circulation and mixing processes in the Liverpool Bay area of the Irish Sea. Geophys. J. Int. 1966, 11, 279–292. [Google Scholar] [CrossRef] [Green Version]
- Bowden, K.F.; Gilligan, R.M. Characteristic features of estuarine circulation as represented in the Mersey Estuary. Limnol. Oceanogr. 1971, 16, 490–502. [Google Scholar] [CrossRef]
- Ruttenberg, K.C.; Berner, R.A. Authigenic apatite formation and burial in sediments from non-upwelling, continental margin environments. Geochim. Cosmochim. Acta 1993, 57, 991–1007. [Google Scholar] [CrossRef]
- Gachter, R.; Muller, B. Why the phosphorus retention of lakes does not necessarily depend on the oxygen supply to their sediment surface. Limnol. Oceanogr. 2003, 48, 929–933. [Google Scholar] [CrossRef]
- Roden, E.E.; Edmonds, J.W. Phosphate mobilization in iron-rich anaerobic sediments: Microbial Fe(III) oxide reduction versus iron-sulfide formation. Arch. Hydrobiol. 1997, 139, 347–378. [Google Scholar] [CrossRef]
- Sugawara, K.; Koyama, T.; Kamata, E. Recovery of precipitated phosphate from lake muds related to sulfate reduction. J. Earth Sci. Nagoya Univ. 1957, 5, 60–67. [Google Scholar]
- Froelich, P.N.; Arthur, M.A.; Burnett, W.C.; Deakin, M.; Hensley, V.; Jahnke, R.; Kaul, L.; Kim, K.H.; Roe, K.; Soutar, A.; et al. Early diagenesis of organic matter in Peru continental margin sediments: Phosphorite precipitation. Mar. Geol. 1988, 80, 309–343. [Google Scholar] [CrossRef]
- Slomp, C.P.; Epping, E.H.G.; Helder, W.; Raaphorst, W.V. A key role for iron-bound phosphorus in authigenic apatite formation in North Atlantic continental platform sediments. J. Mar. Res. 1996, 54, 1179–1205. [Google Scholar] [CrossRef]
- Shulz, H.N.; Schulz, H.D. Large sulfur bacteria and the formation of phosphorite. Science 2005, 307, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Suess, E. Mineral phases formed in anoxic sediments by microbial decomposition of organic matter. Geochim. Cosmochim. Acta 1979, 43, 339–352. [Google Scholar] [CrossRef]
- Egger, M.; Jilbert, T.; Behrends, T.; Rivard, C.; Slomp, C.P. Vivianite is a major sink for phosphorus in methanogenic coastal surface sediments. Geochim. Cosmochim. Acta 2015, 169, 217–235. [Google Scholar] [CrossRef] [Green Version]
- Rothe, M.; Kleeberg, A.; Hupfer, M. The occurrence, identification and environmental relevance of vivianite in waterlogged soils and aquatic sediments. Earth Sci. Rev. 2016, 158, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Lenstra, W.K.; Egger, M.; van Helmond, N.A.G.M.; Kritzberg, E.; Conley, D.J.; Slomp, C.P. Large variations in iron input to an oligotrophic Baltic Sea estuary: Impact on sedimentary phosphorus burial. Biogeosciences 2018, 15, 6979–6996. [Google Scholar] [CrossRef] [Green Version]
- Froelich, P.N.; Bender, M.L.; Luedtke, N.A. The marine phosphorus cycle. Am. J. Sci. 1982, 4, 105. [Google Scholar] [CrossRef]
- Delaney, M.L. Phosphorus accumulation in marine sediments and the oceanic phosphorus cycle. Global Biogeochem. Cycles 1998, 12, 563–572. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Bierkens, M.F.P.; Griffioen, J.; Hefting, M.M.; Middelburg, J.J.; Middelkoop, H.; Slomp, C.P. Nutrient dynamics, transfer and retention along the aquatic continuum from land to ocean: Towards integration of ecological and biogeochemical models. Biogeosciences 2013, 10, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Knowles, R. Denitrification. Microbiol. Rev. 1982, 46, 43–70. [Google Scholar] [CrossRef]
- Seitzinger, S.P. Denitrification in freshwater and coastal marine ecosystems: Ecological and geochemical significance. Limnol. Oceanogr. 1988, 33, 702–724. [Google Scholar] [CrossRef]
- Seitzinger, S. Denitrification in aquatic sediments. In Denitrification in Soil and Sediment; Revsbech, N.P., Sørensen, J., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 301–322. [Google Scholar]
- Smith, C.J.; DeLaune, R.D.; Patrick, W.H., Jr. Fate of riverine nitrate entering an estuary: I. Denitrification and nitrogen burial. Estuaries 1985, 8, 15–21. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Thamdrup, B.; Canfield, D.E. Anaerobic ammonium oxidation (anammox) in the marine environment. Res. Microbiol. 2005, 156, 457–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulin, P.; Pelletier, E.; Saint-Louis, R. Seasonal variability of denitrification efficiency in northern salt marshes: An example from the St. Lawrence Estuary. Mar. Environ. Res. 2007, 63, 490–505. [Google Scholar] [CrossRef] [Green Version]
- Meyer, R.L.; Allen, D.E.; Schmidt, S. Nitrification and denitrification as sources of sediment nitrous oxide production: A microsensor approach. Mar. Chem. 2008, 110, 68–76. [Google Scholar] [CrossRef]
- Senga, Y.; Okumura, M.; Seike, Y. Seasonal and spatial variation in the denitrifying activity in estuarine and lagoonal sediments. J. Oceanogr. 2010, 66, 155–160. [Google Scholar] [CrossRef]
- Christensen, P.B.; Rysgaard, S.; Sloth, N.P.; Dalsgaard, T.; Schwærter, S. Sediment mineralization, nutrient fluxes, denitrification and dissimilatory nitrate reduction to ammonium in an estuarine fjord with sea cage trout farms. Aquat. Microb. Ecol. 2000, 21, 73–84. [Google Scholar] [CrossRef] [Green Version]
- An, S.M.; Gardner, W.S. Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Mar. Ecol. Prog. Ser. 2002, 237, 41–50. [Google Scholar] [CrossRef]
- Nizzoli, D.; Carraro, E.; Nigro, V.; Viaroli, P. Effect of organic enrichment and thermal regime on denitrification and dissimilatory nitrate reduction to ammonium (DNRA) in hypolimnetic sediments of two lowland lakes. Water Res. 2010, 44, 2715–2724. [Google Scholar] [CrossRef]
- Jäntti, H.; Hietanen, S. The effects of hypoxia on sediment nitrogen cycling in the Baltic Sea. Ambio 2012, 41, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Thamdrup, B. New Pathways and processes in the global nitrogen cycle. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 407–428. [Google Scholar] [CrossRef]
- Giblin, A.E.; Tobias, C.R.; Song, B.; Weston, N.; Banta, G.T.; Rivera-Monroy, V.H. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 2013, 26, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Burgin, A.J.; Hamilton, S.K. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Tobias, C.R.; Anderson, I.C.; Canuel, E.A.; Macko, S.A. Nitrogen cycling through a fringing marsh-aquifer ecotone. Mar. Ecol. Prog. Ser. 2001, 210, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Christensen, P.B.; Glud, R.N.; Dalsgaard, T.; Gillespie, P. Impacts of longline mussel farming on oxygen and nitrogen dynamics and biological communities of coastal sediments. Aquaculture 2003, 218, 567–588. [Google Scholar] [CrossRef]
- Ministry of the Environment, Japan. Available online: http://www.env.go.jp/water/heisa.html (accessed on 28 December 2020).
- Ministry of Land, Infrastructure, Transport and Tourism, Japan. Available online: http://www.pa.cgr.mlit.go.jp/chiki/suishitu/ (accessed on 28 December 2020).
- Scheffer, M. Alternative stable states in eutrophic, shallow freshwater systems: A minimal model. Hydrobiol. Bull. 1989, 23, 73–83. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Conley, D.J.; Carstensen, J.; Sanchez-Camacho, M. Return to Neverland: Shifting baselines affect eutrophication restoration targets. Estuar. Coasts 2009, 32, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T. The Set Inlad Sea—Eutrophic or oligotrophic? Mar. Poll. Bull. 2003, 47, 37–42. [Google Scholar] [CrossRef]
- Hiroshima City Agriculture, Forestry and Fisheries Promotion Center. Available online: http://www.haff.city.hiroshima.jp/suisansc/kaki_rekisi.html (accessed on 28 December 2020).
- Wahyudin Yamamoto, T. Modeling bottom-up and top-down controls on the low recruitment success of oyster larvae in Hiroshima Bay, Japan. Aquaculture 2020, 529, 735564. [Google Scholar] [CrossRef]
- Yamamoto, T.; Maeda, H.; Matsuda, O.; Hashimoto, T. Effects of culture density on the growth and fecal production of the oyster Crassostrea gigas. Nippon Suisan Gakkaishi 2009, 75, 230–236, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Ray, N.E.; Fulweiler, R.W. Seasonal patterns of benthic-pelagic coupling in oyster habitats. Mar. Ecol. Prog. Ser. 2020, 652, 95–109. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hashimoto, T.; Matsuda, O.; Go, A.; Nakaguchi, K.; Haraguchi, K. Comparison of sediment quality between Hiroshima Bay and Suo Nada-Special reference to the seasonal variations of sediment quality parameters and their relationships. Nippon Suisan Gakkaishi 2008, 74, 1037–1042, (In Japanese with English Abstract). [Google Scholar] [CrossRef] [Green Version]
- Conley, D.J.; Humborg, C.; Rahm, L.; Savchuk, O.P.; Wulff, F. Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ. Sci. Technol. 2002, 36, 5315–5320. [Google Scholar] [CrossRef]
- Turner, R.E.; Rabalais, N.N.; Justic, D. Gulf of Mexico hypoxia: Alternate states and a legacy. Environ. Sci. Technol. 2008, 42, 2323–2327. [Google Scholar] [CrossRef]
- Hermans, M.; Lenstra, W.K.; van Helmond, N.A.G.M.; Behrends, T.; Egger, M.; Séguret, M.J.; Gustafsson, E.; Gustafsson, B.G.; Slomp, C.P. Impact of natural re-oxygenation on the sediment dynamics of manganese, iron and phosphorus in a euxinic Baltic Sea basin. Geochim. Cosmochim. Acta 2019, 246, 174–196. [Google Scholar] [CrossRef]
- Van Helmond, N.A.; Robertson, E.K.; Conley, D.J.; Hermans, M.; Humborg, C.; Kubeneck, L.J.; Lenstra, W.K.; Slomp, C.P. Removal of phosphorus and nitrogen in sediments of the eutrophic Stockholm archipelago, Baltic Sea. Biogeosciences 2020, 17, 2745–2766. [Google Scholar] [CrossRef]
- Abo, K.; Yamamoto, T. Oligotrophication and its measures in the Seto Inland Sea, Japan. Bull. Jap. Fish. Res. Edu. Agen. 2019, 49, 21–26. [Google Scholar]
- E-Stat, Statistics of Japan. Available online: https://www.e-stat.go.jp/ (accessed on 28 December 2020).
- Ministry of the Environment, Japan. The Partial Revision of the ‘Law Concerning Special Measures for Conservation of the Environment of the Seto Inland Sea’. Available online: https://www.env.go.jp/water/heisa/setonaikai_law_rev.html (accessed on 8 June 2021).
- Ministry of the Environment, Japan. Research on the Basic Information of Environmental Conditions of the Seto Inland Sea, Japan (I); CD-ROM; Ministry of the Environment: Tokyo, Japan, 1988. (In Japanese)
- Ministry of the Environment, Japan. Research on the Basic Information of Environmental Conditions of the Seto Inland Sea, Japan (II); CD-ROM; Ministry of the Environment: Tokyo, Japan, 1997. (In Japanese)
- Ministry of the Environment, Japan. Research on the Basic Information of Environmental Conditions of the Seto Inland Sea, Japan (III); CD-ROM; Ministry of the Environment: Tokyo, Japan, 2006. (In Japanese)
- Ministry of the Environment, Japan. The Methods of Sediment Analyses. 1998. Available online: http://www.env.go.jp/hourei/05/000178.html (accessed on 28 December 2020).
- Yamamoto, T.; Yoshikawa, S.; Hashimoto, T.; Takasugi, Y.; Matsuda, O. Estuarine circulation processes in the northern Hiroshima Bay, Japan. Bull. Coast. Oceanogr. 2000, 37, 111–118, (In Japanese with English abstract). [Google Scholar]
- Yamamoto, H.; Yamamoto, T.; Takada, T.; Mito, Y.; Takahashi, T. Dynamic analysis of oxygen-deficient water mass formed in the northern part of Hiroshima Bay using a pelagic-benthic coupled ecosystem model. J. Jap. Soc. Water Envir. 2011, 34, 19–28, (In Japanese with English Abstract). [Google Scholar] [CrossRef] [Green Version]
- Nishimura, K.; Sayanagi, K.; Nagao, T.; Murakami, F.; Joshima, M.; Takatou, M. Digitization of an analog sub-bottom profiler. J. Sch. Mar. Sci. Tech. Tokai Univ. 2008, 6, 129–139, (In Japanese with English Abstract). [Google Scholar]
- Arakawa, K. Teishitsu Chousa Hou (Methods of Sediment Quality Analyses). In Suishitsu Odaku Chosa Shishin; Japanese Fishery Resource Conservation Association, Ed.; Japanese Fishery Resource Conservation Association: Tokyo, Japan, 1980; pp. 237–272. (In Japanese) [Google Scholar]
- Rickard, D.; Morse, J.W. Acid volatile sulfide (AVS). Mar. Chem. 2005, 97, 141–197. [Google Scholar] [CrossRef]
- Asaoka, S.; Yamamoto, T.; Hayakawa, S. Removal of hydrogen sulfide using granulated coal ash. J. Japan Soc. Wat. Environ. 2009, 32, 363–368, (In Japanese with English Abstract). [Google Scholar] [CrossRef] [Green Version]
- Asaoka, S.; Yamamoto, T.; Takahashi, Y.; Yamamoto, H.; Kim, K.H.; Orimoto, K. Development of an on-site simplified determination method for hydrogen sulfide in marine sediment pore water using a shipboard ion electrode with consideration of hydrogen sulfide oxidation rate. In Interdisciplinary Studies on Environmental Chemistry—Environmental Pollution and Ecotoxicology; Kawaguchi, M., Misaki, K., Sato, H., Yokokawa, T., Itai, T., Nguyen, T.M., Ono, J., Tanabe, S., Eds.; TERRAPUB: Tokyo, Japan, 2012; pp. 345–352. [Google Scholar]
- Japan Fisheries Resource Conservation Association. Standard for Fisheries Water; Japanese Fishery Resource Conservation Association: Tokyo, Japan, 1995; 68p. (In Japanese) [Google Scholar]
- Kittiwanich, J.; Yamamoto, T.; Kawaguchi, O.; Hashimoto, T. Analyses of phosphorus and nitrogen cyclings in the estuarine ecosystem of Hiroshima Bay by a pelagic and benthic coupled model. Est. Coast. Shelf Sci. 2007, 75, 189–204. [Google Scholar] [CrossRef]
- Hiroshima Fisheries and Marine Technology Center. Available online: https://www.pref.hiroshima.lg.jp/soshiki/32/suigi-top.html (accessed on 31 May 2021).
- Kim, D.H.; Matsuda, O.; Yamamoto, T. Nitrification, denitrification and nitrate reduction rates in the sediment of Hiroshima Bay, Japan. J. Oceanogr. 1997, 53, 317–324. [Google Scholar]
- Yamamoto, T.; Takeshita, K.; Hiraga, N.; Hashimoto, T. An estimation of net ecosystem metabolism and net denitrification of the Seto Inland Sea, Japan. Ecol. Model. 2008, 215, 55–68. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kubo, A.; Hashimoto, T.; Nishii, Y. Long-term changes in net ecosystem metabolism and net denitrification in the Ohta River estuary of northern Hiroshima Bay—An analysis based on the phosphorus and nitrogen budgets. In Progress in Aquatic Ecosystem Research; Burk, A.R., Ed.; Nova Science Publisher, Inc.: New York, NY, USA, 2005; pp. 99–120. [Google Scholar]
- Kuroda, A.; Takiguchi, N.; Kato, J.; Ohtake, H. Development of technolgies to save phosphorus resources in response to phosphate crisis. J. Environ. Biotech. 2005, 4, 87–94. (In Japanese) [Google Scholar]
- Ministry of the Environment, Japan. Environmental Technology Verification (ETV) Program. Available online: https://www.env.go.jp/policy/etv/ (accessed on 4 June 2021).
- Asaoka, S.; Yamamoto, T.; Kondo, S.; Hayakawa, S. Removal of hydrogen sulfide using crushed oyster shell from pore water to remediate organically enriched coastal marine sediments. Biores. Technol. 2009, 100, 4127–4132. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Kondo, S.; Kim, K.H.; Asaoka, S.; Yamamoto, H.; Tokuoka, M.; Hibino, T. Remediation of muddy tidal flat sediments using hot air-dried crushed oyster shells. Mar. Poll. Bull. 2012, 64, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, S.; Yamamoto, T. Blast furnace slag can effectively remediate coastal marine sediments affected by organic enrichment. Mar. Poll. Bull. 2010, 60, 573–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Asaoka, S.; Yamamoto, T.; Hayakawa, S.; Takeda, K.; Katayama, M.; Onoue, T. Mechanisms of hydrogen sulfide removal with steel making slag. Environ. Sci. Technol. 2012, 46, 10169–10174. [Google Scholar] [CrossRef]
- Asaoka, S.; Yamamoto, T.; Yoshioka, I.; Tanaka, H. Remediation of coastal marine sediments using granulated coal ash. J. Hazard. Mat. 2009, 172, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Asaoka, S.; Hayakawa, S.; Kim, K.H.; Takeda, K.; Katayama, M.; Yamamoto, T. Combined adsorption and oxidation mechanisms of hydrogen sulfide on granulated coal ash. J. Coll. Interface Sci. 2012, 377, 284–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, Y. Outlook of Japan’s policy in recycling phosphorus from sewage. J. Japan Soc. Wat. Environ. 2011, 34, 2–6. (In Japanese) [Google Scholar]
- Abelson, P.H. A potential phosphate crisis. Science 1999, 283, 2015. [Google Scholar] [CrossRef]
- Komai, Y. Changes of sediment quality and benthos in the Seto Inland Sea. In Sediment Quality of the Seto Inland Sea, Japan; Yanagi, T., Ed.; Kouseisha-Kouseikaku: Tokyo, Japan, 2008; pp. 43–60. (In Japanese) [Google Scholar]
- Ingall, E.D.; Bustin, R.M.; Vancappellen, P. Influence of water column anoxia on the burial and preservation of carbon and phosphorus in marine shales. Geochim. Cosmochim. Acta 1993, 57, 303–316. [Google Scholar] [CrossRef]
- Rozan, T.F.; Taillefert, M.; Trouwborst, R.E.; Glazer, B.T.; Ma, S.F.; Herszage, J.; Valdes, L.M.; Price, K.S.; Luther, G.W. Iron-sulfur-phosphorus cycling in the sediments of a shallow coastal bay: Implications for sediment nutrient release and benthic macroalgal blooms. Limnol. Oceanogr. 2002, 47, 1346–1354. [Google Scholar] [CrossRef] [Green Version]
- Heidenreich, M.; Kleeberg, A. Phosphorus-binding in iron-rich sediments of a shallow Reservoir: Spatial characterization based on sonar data. Hydrobiologia 2003, 506, 147–153. [Google Scholar] [CrossRef]
- Hiroshima Prefecture: Records of Huge Desarsters in Hiroshima Area. Available online: https://www.sabo.pref.hiroshima.lg.jp/portal/sonota/saigai/002dosya.htm (accessed on 20 June 2021).
- Yamamoto, T.; Hanazato, T. Oligotrophication in Seas and Lakes—No Fish. in Clear Water; Yamamoto, T., Hanazato, T., Eds.; Chijin Shokan: Tokyo, Japan, 2015; p. 195. (In Japanese) [Google Scholar]
- Harada, K.; Abo, K.; Kawasaki, S.; Takesako, F.; Miyahara, K. Influence of DIN derived from ports and treated sewage effluent on nori (Pyropia) farms of the northeastern part of Harima Nada. Bull. Jpn. Soc. Fish. Oceanogr. 2018, 82, 26–35, (In Japanese with English Abstract). [Google Scholar]
- Abo, K.; Tarutani, K.; Harada, K.; Miyahara, K.; Nakayama, A.; Yagi, H. Effects of nutrient discharge on Nori aquaculture area in Kako River estuary. J. JSCE Ser. B2 Coast. Eng. 2012, 68, I1116–I1120, (In Japanese with English Abstract). [Google Scholar]
- House of Representatives, Japan. The Diet, No. 204, Report on Discussions of The Partial Revision of the ‘Law Concerning Special Measures for Conservation of the Environment of the Seto Inland Sea’. Available online: https://www.shugiin.go.jp/internet/itdb_gian.nsf/html/gian/keika/1DD1E7A.htm (accessed on 9 June 2021).
- Yamamoto, T.; Ishida, S.; Nakahara, S.; Hiraoka, K.; Omichi, Y.; Mutsuda, H. Fertilizer application to enhance the growth of raft-cultured oysters. In Proceedings of the JSFS 85th Anniversary-Commemorative International Symposium “Fisheries Science for Future Generations”, Tokyo, Japan, 22–24 September 2017. No. 05001, 2p. [Google Scholar]
- Yamamoto, H.; Yamamoto, T.; Mito, Y.; Asaoka, S. Numerical evaluation of the use of granulated coal ash to reduce an oxygen-deficient water mass. Mar. Poll. Bull. 2016, 107, 188–205. [Google Scholar] [CrossRef] [PubMed]








| Depth (cm) | TOC (mg C g dry−1) | TN (mg N g dry−1) | TS (mg S g dry−1) | TP (mg P g dry−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avg | Stdev | CV (%) | Avg | Stdev | CV (%) | Avg | Stdev | CV (%) | Avg | Stdev | CV (%) | |
| 2.5 | 15 | 3 | 17 | 1.6 | 0.5 | 32 | 3.9 | 1.4 | 35 | 0.57 | 0.04 | 6 |
| 7.5 | 14 | 3 | 21 | 1.4 | 0.4 | 31 | 3.9 | 1.3 | 32 | 0.52 | 0.04 | 9 |
| 12.5 | 14 | 3 | 24 | 1.2 | 0.4 | 33 | 4.1 | 1.7 | 41 | 0.50 | 0.06 | 11 |
| 17.5 | 13 | 3 | 25 | 1.2 | 0.4 | 35 | 3.7 | 1.6 | 43 | 0.46 | 0.03 | 6 |
| 25 | 12 | 4 | 30 | 1.2 | 0.4 | 36 | 3.6 | 1.3 | 37 | 0.46 | 0.04 | 8 |
| Parameters | Area | Value Range |
|---|---|---|
| Eh (mV) | N | −150–−27 |
| S | −153–+45 | |
| WC (%) | N | 69.8–82.5 |
| S | 46.6–81.8 | |
| IL (%) | N | 10.9–12.7 |
| S | 4.8–11.3 | |
| AVS (mg g dw−1) | N | 0.17–1.14 |
| S | 0.04–0.35 | |
| H2S (mg L−1) | N | 0–19 |
| S | 0–23 |
| Year | TOC (mg g−1) | TN (mg g−1) | TP (mg g−1) | References | |
|---|---|---|---|---|---|
| 1982 | Max | 30 | 3.5 | 0.75 | [54] |
| Min | 9.6 | 1.0 | 0.34 | ||
| Avg | 20 | 2.5 | 0.59 | ||
| SD | 5.4 | 0.66 | 0.11 | ||
| CV (%) | 27 | 27 | 18 | ||
| 1993 | Max | 26 | 2.8 | 0.62 | [55] |
| Min | 2.8 | 0.36 | 0.26 | ||
| Avg | 18 | 2.1 | 0.53 | ||
| SD | 5.7 | 0.62 | 0.090 | ||
| CV (%) | 32 | 30 | 17 | ||
| 2003 | Max | 29 | 3.1 | 0.68 | [56] |
| Min | 4.4 | 0.60 | 0.20 | ||
| Avg | 17 | 2.2 | 0.51 | ||
| SD | 6.8 | 0.78 | 0.12 | ||
| CV (%) | 39 | 36 | 23 | ||
| Oct 2020 | Max | 21 | 3.0 | 0.66 | This study |
| Min | 9.4 | 0.62 | 0.49 | ||
| Avg | 15 | 1.6 | 0.57 | ||
| SD | 2.6 | 0.50 | 0.040 | ||
| CV (%) | 17 | 32 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, T.; Orimoto, K.; Asaoka, S.; Yamamoto, H.; Onodera, S.-i. A Conflict between the Legacy of Eutrophication and Cultural Oligotrophication in Hiroshima Bay. Oceans 2021, 2, 546-565. https://doi.org/10.3390/oceans2030031
Yamamoto T, Orimoto K, Asaoka S, Yamamoto H, Onodera S-i. A Conflict between the Legacy of Eutrophication and Cultural Oligotrophication in Hiroshima Bay. Oceans. 2021; 2(3):546-565. https://doi.org/10.3390/oceans2030031
Chicago/Turabian StyleYamamoto, Tamiji, Kaori Orimoto, Satoshi Asaoka, Hironori Yamamoto, and Shin-ichi Onodera. 2021. "A Conflict between the Legacy of Eutrophication and Cultural Oligotrophication in Hiroshima Bay" Oceans 2, no. 3: 546-565. https://doi.org/10.3390/oceans2030031