Quercitrin from Toona sinensis (Juss.) M.Roem. Attenuates Acetaminophen-Induced Acute Liver Toxicity in HepG2 Cells and Mice through Induction of Antioxidant Machinery and Inhibition of Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Quercitrin
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. ROS Formation Assay
2.6. Assay of Reporter Gene Activity
2.7. Animals and Experimental Design
2.8. Measurement of Plasma ALT and AST Levels
2.9. Assay for Antioxidant Enzymes Activity
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Protective Effect of Quercitrin against Oxidative Stress in APAP-Induced HepG2 Cells
3.2. Upregulation of Nrf2/ARE-Mediated Phase II Detoxifying Enzymes by Quercitrin in APAP-Treated HepG2 Cells
3.3. Protective Effect of Quercitrin against APAP-Induced Hepatotoxicity in Vivo
3.4. Protective Effect of Quercitrin against APAP-Induced Hepatotoxicity through Enhancement of Activity and Expression of Antioxidant Enzymes
3.5. Upregulation of NQO1 Expression by Nrf2-Mediated Quercitrin in APAP-Treated Mice
3.6. Quercitrin Attenuates Inflammatory Response in APAP-Treated Mice
3.7. Activation of Cytoprotective Mechanisms by Quercitrin through Regulation of MAPK Pathways in APAP-Treated Mice
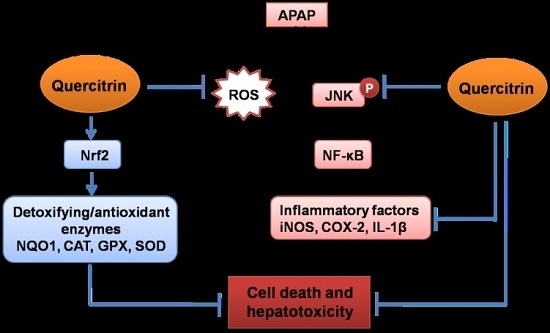
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APAP | Acetaminophen |
| ALT | Alanine transaminase |
| ARE | Antioxidant response element |
| AST | Aspartate aminotransferase |
| CAT | Catalase |
| COX-2 | Cyclooxygenase-2 |
| CYP | Cytochrome P450 |
| ERK | Extracellular signal regulated kinase |
| GSH | Glutathione |
| GPx | Glutathione peroxidase |
| iNOS | Inducible nitric oxide synthase |
| JNK | c-Jun N-terminal kinase |
| Keap 1 | Kelch-like ECH-associated protein 1 |
| MAPK | Mitogen activated protein kinase |
| NAC | N-acetylcysteine |
| NQO1 | NADPH:quinone oxidoreductase 1 |
| Nrf2 | Nuclear factor erythroid-2-related factor-2 |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
References
- Slitt, A.M.L.; Dominick, P.K.; Roberts, J.C.; Cohen, S.D. Effect of ribose cysteine pretreatment on hepatic and renal acetaminophen metabolite formation and glutathione depletion. Basic Clin. Pharmacol. Toxicol. 2005, 96, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Fu, J.; Xia, X.; Su, C.; Song, Y. Bazhen decoction protects against acetaminophen induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. PLoS ONE 2014, 9, e107405. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.-R.; Kim, Y.-H.; Hwang, J.H.; Choi, D.-H.; Kim, K.-S.; Oh, W.-K.; Lee, C.-H. Sulforaphane protects against acetaminophen-induced hepatotoxicity. Food Chem. Toxicol. 2015, 80, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Williams, C.D.; Ramachandran, A.; Bajt, M.L. Acetaminophen hepatotoxicity and repair: The role of sterile inflammation and innate immunity. Liver Int. 2012, 32, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.A.; Whyte, I.M.; O’Connell, D.L.; Dawson, A.H. Oral or intravenous N-acetylcysteine: Which is the treatment of choice for acetaminophen (paracetamol) poisoning? J. Toxicol. Clin. Toxicol. 1999, 37, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, R.J.; Meredith, T.J. Use of N-acetylcysteine in clinical toxicology. Am. J. Med. 1991, 91, 131S–139S. [Google Scholar] [CrossRef]
- Heard, K.J. Acetylcysteine for acetaminophen poisoning. N. Engl. J. Med. 2008, 359, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, E.A.; Bateman, D.N. Adverse reactions associated with acetylcysteine. Clin. Toxicol. 2009, 47, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.-J.; Jeong, J.-H.; Kang, H.-S.; Jin, K.-S.; Jun, M.; Jeong, W.-S. Stimulation of activity and expression of antioxidant enzymes by solvent fractions and isolated compound from Cedrela sinensis leaves in HepG2 cells. J. Med. Food 2011, 14, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.-C.; Rajbanshi, R.; Calhoun, C.; Chiu, R.H. Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules 2010, 15, 8377–8389. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, C.-Y.; Hseu, Y.-C.; Chang, Y.-C.; Kumar, K.J.S.; Ho, T.-Y.; Yang, H.-L. Toona sinensis and its major bioactive compound gallic acid inhibit LPS-induced inflammation in nuclear factor-κB transgenic mice as evaluated byin vivo bioluminescence imaging. Food Chem. 2013, 136, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-F.; Yang, Y.-C.; Hsu, H.-K.; Hwang, S.-L.; Lee, K.-S.; Lieu, A.-S.; Chan, T.-F.; Lin, C.-L. Toona sinensis leaf extract has antinociceptive effect comparable with non-steroidal anti-inflammatory agents in mouse writhing test. BMC Complement. Altern. Med. 2015, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.R.; Maquiaveli, C.D.C.; Magalhães, P.P. The leishmanicidal flavonols quercetin and quercitrin target Leishmania (Leishmania) amazonensis arginase. Exp. Parasitol. 2012, 130, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Fachinetto, R.; Dalla Corte, C.L.; Brito, V.B.; Severo, D.; de Oliveira Costa Dias, G.; Morel, A.F.; Nogueira, C.W.; Rocha, J.B.T. Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res. 2006, 1107, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ader, P.; Blöck, M.; Pietzsch, S.; Wolffram, S. Interaction of quercetin glucosides with the intestinal sodium/glucose co-transporter (SGLT-1). Cancer Lett. 2001, 162, 175–180. [Google Scholar] [CrossRef]
- Gee, J.M.; DuPont, M.S.; Rhodes, M.J.C.; Johnson, I.T. Quercetin glucosides interact with the intestinal glucose transport pathway 1. Free Radic. Biol. Med. 1998, 25, 19–25. [Google Scholar] [CrossRef]
- Babujanarthanam, R.; Kavitha, P.; Mahadeva Rao, U.S.; Pandian, M. Quercitrin a bioflavonoid improves the antioxidant status in streptozotocin: Induced diabetic rat tissues. Mol. Cell. Biochem. 2011, 358, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ham, Y.-M.; Yoon, W.-J.; Park, S.-Y.; Song, G.-P.; Jung, Y.-H.; Jeon, Y.-J.; Kang, S.-M.; Kim, K.-N. Quercitrin protects against oxidative stress-induced injury in lung fibroblast cells via up-regulation of Bcl-xl. J. Funct. Foods 2012, 4, 253–262. [Google Scholar] [CrossRef]
- Camuesco, D.; Comalada, M.; Rodríguez-Cabezas, M.E.; Nieto, A.; Lorente, M.D.; Concha, A.; Zarzuelo, A.; Gálvez, J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br. J. Pharmacol. 2004, 143, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Tai, B.; Cuong, N.; Kim, Y.-H.; Jang, H.-D. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum). Food Sci. Biotechnol. 2012, 21, 587–595. [Google Scholar] [CrossRef]
- Cruz, E.A.; Da-Silva, S.A.G.; Muzitano, M.F.; Silva, P.M.R.; Costa, S.S.; Rossi-Bergmann, B. Immunomodulatory pretreatment with Kalanchoe pinnata extract and its quercitrin flavonoid effectively protects mice against fatal anaphylactic shock. Int. Immunopharmacol. 2008, 8, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-M.; Ham, Y.-M.; Yoon, W.-J.; Roh, S.W.; Jeon, Y.-J.; Oda, T.; Kang, S.-M.; Kang, M.-C.; Kim, E.-A.; Kim, D.; et al. Quercitrin protects against ultraviolet B-induced cell death in vitro and in anin vivo zebrafish model. J. Photochem. Photobiol. B 2012, 114, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, W.; Son, Y.-O.; Sun, L.; Lu, J.; Kim, D.; Wang, X.; Yao, H.; Wang, L.; Pratheeshkumar, P.; et al. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol. Appl. Pharmacol. 2013, 269, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Cincin, Z.B.; Unlu, M.; Kiran, B.; Bireller, E.S.; Baran, Y.; Cakmakoglu, B. Molecular mechanisms of quercitrin-induced apoptosis in non-small cell lung cancer. Arch. Med. Res. 2014, 45, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Chen, C.; Mo, Y.-Y.; Hebbar, V.; Owuor, E.D.; Tan, T.-H.; Kong, A.-N.T. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J. Biol. Chem. 2000, 275, 39907–39913. [Google Scholar] [CrossRef] [PubMed]
- Bektur, N.E.; Sahin, E.; Baycu, C.; Unver, G. Protective effects of silymarin against acetaminophen-induced hepatotoxicity and nephrotoxicity in mice. Toxicol. Ind. Health 2016, 32, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Bogdanska, J.J.; Korneti, P.; Todorova, B. Erythrocyte superoxide dismutase, glutathione peroxidase and catalase activities in healthy male subjects in republic of macedonia. Bratisl. Lek. Listy 2003, 104, 108–114. [Google Scholar] [PubMed]
- Oyanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Lessig, J.; Fuchs, B. Plasmalogens in biological systems: Their role in oxidative processes in biological membranes, their contribution to pathological processes and aging and plasmalogen analysis. Curr. Med. Chem. 2009, 16, 2021–2041. [Google Scholar] [CrossRef] [PubMed]
- Mena, S.; Ortega, A.; Estrela, J.M. Oxidative stress in environmental-induced carcinogenesis. Mutat. Res. 2009, 674, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; McGill, M.R.; Williams, C.D.; Ramachandran, A. Current issues with acetaminophen hepatotoxicity—A clinically relevant model to test the efficacy of natural products. Life Sci. 2011, 88, 737–745. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Williams, C.D.; Xie, Y.; Ramachandran, A.; Jaeschke, H. Acetaminophen-induced liver injury in rats and mice: Comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 2012, 264, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Itoh, K.; Nagayoshi, E.; Haruta, J.; Kimura, T.; O’Connor, T.; Harada, T.; Yamamoto, M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001, 59, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Han, X.-D.; Kan, Y.W. An important function of Nrf2 in combating oxidative stress: Detoxification of acetaminophen. Proc. Natl. Acad. Sci. USA 2001, 98, 4611–4616. [Google Scholar] [CrossRef] [PubMed]
- Aleksunes, L.M.; Manautou, J.E. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicol. Pathol. 2007, 35, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Motohashi, H.; Kobayashi, A.; Aburatani, H.; Kensler, T.W.; Yamamoto, M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 2006, 339, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kim, Y.-H.; Noh, J.-R.; Gang, G.-T.; Kim, K.-S.; Chung, H.; Tadi, S.; Yim, Y.-H.; Shong, M.; Lee, C.-H. The protective role of NAD(P)H:Quinone oxidoreductase 1 on acetaminophen-induced liver injury is associated with prevention of adenosine triphosphate depletion and improvement of mitochondrial dysfunction. Arch. Toxicol. 2015, 89, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Moffit, J.S.; Aleksunes, L.M.; Kardas, M.J.; Slitt, A.L.; Klaassen, C.D.; Manautou, J.E. Role of NAD(P)H:Quinone oxidoreductase 1 in clofibrate-mediated hepatoprotection from acetaminophen. Toxicology 2007, 230, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.A.; Shelar, S.B.; Dang, T.-M.; Lee, B.N.-C.; Yang, H.; Ong, S.-M.; Ng, H.-L.; Chui, W.-K.; Wong, S.-C.; Chew, E.-H. Sulforaphane and its methylcarbonyl analogs inhibit the LPS-stimulated inflammatory response in human monocytes through modulating cytokine production, suppressing chemotactic migration and phagocytosis in a NF-κB- and MAPK-dependent manner. Int. Immunopharmacol. 2015, 24, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, A.-C.; Sun, R.; Mishin, V.; Hall, L.B.; Laskin, J.D.; Laskin, D.L. Role of galectin-3 in acetaminophen-induced hepatotoxicity and inflammatory mediator production. Toxicol. Sci. 2012, 127, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-W.; Lee, H.-S.; Jung, K.; Lee, H.; Hong, S.-S. Protective effect of fucoidan against acetaminophen-induced liver injury. Arch. Pharm. Res. 2012, 35, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.; Zhang, J.; Song, S.; Miao, R.; Liu, S.; Pang, Q.; Wu, Q.; Liu, C. Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int. Immunopharmacol. 2015, 27, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Farhood, A.; Jaeschke, H. Role of caspase-1 and interleukin-1β in acetaminophen-induced hepatic inflammation and liver injury. Toxicol. Appl. Pharmacol. 2010, 247, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kong, A.-N.T. Dietary cancer-chemopreventive compounds: From signaling and gene expression to pharmacological effects. Trends Pharmacol. Sci. 2005, 26, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, N.; Shinohara, M.; Saberi, B.; Gaarde, W.A.; Han, D.; Kaplowitz, N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008, 283, 13565–13577. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, B.K.; Liu, Z.X.; Han, D.; Hanawa, N.; Gaarde, W.A.; Kaplowitz, N. C-jun n-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 2006, 131, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Saito, C.; Lemasters, J.J.; Jaeschke, H. C-jun n-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010, 246, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Pollock, K.J.; Frew, J.; Mackinnon, A.C.; Flavell, R.A.; Davis, R.J.; Sethi, T.; Simpson, K.J. Critical role of c-jun (NH2) terminal kinase in paracetamol-induced acute liver failure. Gut 2007, 56, 982–990. [Google Scholar] [CrossRef] [PubMed]








| Groups | Treatment | Initial Body Weight (g) | Final Body Weight (g) | Liver Weight (g) | Relative Liver Weight (Percentage Ratio) |
|---|---|---|---|---|---|
| I | Control | 20.12 ± 0.49 | 23.02 ± 1.36 | 1.42 ± 0.09 | 6.19 ± 0.30 |
| II | Quercitrin (50 mg/kg) | 19.62 ± 0.59 | 23.40 ± 0.45 | 1.53 ± 0.11 | 6.55 ± 0.53 |
| III | APAP (300 mg/kg) | 19.68 ± 0.40 | 23.37 ± 0.90 | 1.65 ± 0.11 | 7.21 ± 0.36 # |
| IV | Quercitrin (10 mg/kg) + APAP | 20.20 ± 0.34 | 24.1 ± 0.41 | 1.50 ± 0.10 | 6.20 ± 0.31 * |
| V | Quercitrin (50 mg/kg) + APAP | 19.92 ± 0.38 | 23.02 ± 0.97 | 1.47 ± 0.09 | 6.40 ± 0.28 * |
| VI | Silymarin (50 mg/kg) + APAP | 19.68 ± 0.85 | 23.42 ± 0.92 | 1.50 ± 0.10 | 6.38 ± 0.19 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, V.-L.; Ko, S.-Y.; Jun, M.; Jeong, W.-S. Quercitrin from Toona sinensis (Juss.) M.Roem. Attenuates Acetaminophen-Induced Acute Liver Toxicity in HepG2 Cells and Mice through Induction of Antioxidant Machinery and Inhibition of Inflammation. Nutrients 2016, 8, 431. https://doi.org/10.3390/nu8070431
Truong V-L, Ko S-Y, Jun M, Jeong W-S. Quercitrin from Toona sinensis (Juss.) M.Roem. Attenuates Acetaminophen-Induced Acute Liver Toxicity in HepG2 Cells and Mice through Induction of Antioxidant Machinery and Inhibition of Inflammation. Nutrients. 2016; 8(7):431. https://doi.org/10.3390/nu8070431
Chicago/Turabian StyleTruong, Van-Long, Se-Yeon Ko, Mira Jun, and Woo-Sik Jeong. 2016. "Quercitrin from Toona sinensis (Juss.) M.Roem. Attenuates Acetaminophen-Induced Acute Liver Toxicity in HepG2 Cells and Mice through Induction of Antioxidant Machinery and Inhibition of Inflammation" Nutrients 8, no. 7: 431. https://doi.org/10.3390/nu8070431