Relationship between Dietary Patterns with Benign Prostatic Hyperplasia and Erectile Dysfunction: A Collaborative Review
Abstract
:1. Introduction
2. Materials and Methods
3. Erectile Dysfunction
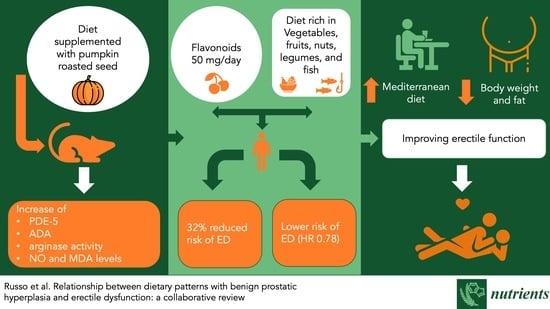
3.1. Studies in Animal Models
3.2. Studies in Humans
4. Benign Prostatic Hyperplasia
4.1. Studies in Animal Models
4.2. Studies in Human
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Russo, G.I.; Cimino, S.; Fragalà, E.; Privitera, S.; La Vignera, S.; Condorelli, R.; Calogero, A.E.; Castelli, T.; Favilla, V.; Morgia, G. Insulin resistance is an independent predictor of severe lower urinary tract symptoms and of erectile dysfunction: Results from a cross-sectional study. J. Sex. Med. 2014, 11, 2074–2082. [Google Scholar] [CrossRef]
- Russo, G.I.; Castelli, T.; Urzì, D.; Privitera, S.; La Vignera, S.; Condorelli, R.A.; Calogero, A.E.; Favilla, V.; Cimino, S.; Morgia, G. Emerging links between non-neurogenic lower urinary tract symptoms secondary to benign prostatic obstruction, metabolic syndrome and its components: A systematic review. Int. J. Urol. 2015, 22, 982–990. [Google Scholar] [CrossRef]
- Russo, G.I.; Campisi, D.; Mauro, M.D.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary consumption of phenolic acids and prostate cancer: A case-control study in sicily, Southern Italy. Molecules 2017, 22, 2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, G.I.; Di Mauro, M.; Regis, F.; Reale, G.; Campisi, D.; Marranzano, M.; Giudice, A.L.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Association between dietary phytoestrogens intakes and prostate cancer risk in Sicily. Aging Male 2018, 21, 48–54. [Google Scholar] [CrossRef]
- Reale, G.; Russo, G.I.; Di Mauro, M.; Regis, F.; Campisi, D.; Giudice, A.L.; Marranzano, M.; Ragusa, R.; Castelli, T.; Cimino, S.; et al. Association between dietary flavonoids intake and prostate cancer risk: A case-control study in Sicily. Complement Ther. Med. 2018, 39, 14–18. [Google Scholar] [CrossRef]
- McKinlay, J.B. The worldwide prevalence and epidemiology of erectile dysfunction. Int. J. Impot. Res. 2000, 12 (Suppl. S4), S6–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, G.I.; di Mauro, M.; Cocci, A.; Cacciamani, G.; Cimino, S.; Serefoglu, E.C.; Albersen, M.; Capogrosso, P.; Fode, M.; Verze, P. Consulting “Dr Google” for sexual dysfunction: A contemporary worldwide trend analysis. Int. J. Impot. Res. 2019, 32, 455–461. [Google Scholar] [CrossRef]
- Salonia, A.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Sexual and Reproductive Health—2021 Update: Male Sexual Dysfunction. Eur. Urol. 2021, 80, 333–357. [Google Scholar] [CrossRef]
- Kessler, A.; Sollie, S.; Challacombe, B.; Briggs, K.; Van Hemelrijck, M. The global prevalence of erectile dysfunction: A review. BJU Int. 2019, 124, 587–599. [Google Scholar] [CrossRef]
- De Nunzio, C.; Roehrborn, C.G.; Andersson, K.-E.; McVary, K.T. Erectile Dysfunction and Lower Urinary Tract Symptoms. Eur. Urol. Focus 2017, 3, 352–363. [Google Scholar] [CrossRef] [PubMed]
- ElJalby, M.; Thomas, D.; Elterman, D.; Chughtai, B. The effect of diet on BPH.; LUTS and ED. World J. Urol. 2019, 37, 1001–1005. [Google Scholar] [CrossRef]
- Kalter-Leibovici, O.; Wainstein, J.; Ziv, A.; Harman-Bohem, I.; Murad, H.; Raz, I. Clinical, socioeconomic, and lifestyle parameters associated with erectile dysfunction among diabetic men. Diabetes Care 2005, 28, 1739–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacon, C.G.; Mittleman, M.A.; Kawachi, I.; Giovannucci, E.; Glasser, D.B.; Rimm, E.B. Sexual Function in Men Older Than 50 Years of Age: Results from the Health Professionals Follow-up Study. Ann. Intern. Med. 2003, 139, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Musicki, B.; Liu, T.; Strong, T.; Jin, L.; Laughlin, M.H.; Turk, J.R.; Burnett, A.L. Low-fat diet and exercise preserve eNOS regulation and endothelial function in the penis of early atherosclerotic pigs: A molecular analysis. J. Sex. Med. 2008, 5, 552–561. [Google Scholar] [CrossRef] [Green Version]
- Esposito, K.; Giugliano, F.; Maiorino, M.I.; Giugliano, D. Dietary Factors, Mediterranean Diet and Erectile Dysfunction. J. Sex. Med. 2010, 7, 2338–2345. [Google Scholar] [CrossRef]
- Akomolafe, S.F.; Olasehinde, T.A.; Aluko, B.T. Diets supplemented with raw and roasted pumpkin (Cucurbita pepo L) seeds improved some biochemical parameters associated with erectile function in rats. J. Food Biochem. 2021, 45, e13629. [Google Scholar] [CrossRef]
- Akomolafe, S.F.; Oboh, G.; Oyeleye, S.I.; Molehin, O.R.; Ogunsuyi, O.B. Phenolic composition and inhibitory ability of methanolic extract from pumpkin (Cucurbita pepo L) seeds on Fe-induced thiobarbituric acid reactive species in albino rat’s testicular tissue in-vitro. J. Appl. Pharm. Sci. 2016, 6, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Robson, S.C.; Sévigny, J.; Zimmermann, H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal 2006, 2, 409–430. [Google Scholar] [CrossRef] [Green Version]
- Sträter, N. Ecto-5′-nucleotidase: Structure function relationships. Purinergic Signal 2006, 2, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, M.R.; Kellems, R.E. Adenosine deaminase deficiency: Metabolic basis of immune deficiency and pulmonary inflammation. Adv. Immunol. 2005, 86, 1–41. [Google Scholar] [CrossRef]
- Olabiyi, A.A.; Carvalho, F.B.; Bottari, N.B.; Lopes, T.F.; da Costa, P.; Stefanelo, N.; Morsch, V.M.; Akindahunsi, A.A.; Oboh, G.; Schetinger, M.R. Dietary supplementation of tiger nut alters biochemical parameters relevant to erectile function in L-NAME treated rats. Food Res. Int. 2018, 109, 358–367. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, X.; Dai, Y.; Zhang, Y.; Tang, Y.; Sun, H.; Mi, T.; Kellems, R.E.; Blackburn, M.R.; Xia, Y. Adenosine Deaminase Enzyme Therapy Prevents and Reverses the Heightened Cavernosal Relaxation in Priapism. J. Sex. Med. 2010, 7, 3011–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olabiyi, A.A.; AlliSmith, Y.R.; Ukwenya, V.O. Quercetin enhances sexual behavior and improves ectonucleotidases activity in the hypothalamus of rats treated with cyclosporine. J. Food Biochem. 2021, 45, e13864. [Google Scholar] [CrossRef]
- Eleazu, C.; Obianuju, N.; Eleazu, K.; Kalu, W. The role of dietary polyphenols in the management of erectile dysfunction–Mechanisms of action. Biomed. Pharmacother. 2017, 88, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Cheitlin, M.D. Erectile dysfunction: The earliest sign of generalized vascular disease? J. Am. Coll. Cardiol. 2004, 43, 185–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minhas, S.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2021 Update on Male Infertility. Eur. Urol. 2021, 80, 603–620. [Google Scholar] [CrossRef]
- Djordjevic, D.; Vukovic, I.; Milenkovic Petronic, D.; Radovanovic, G.; Seferovic, J.; Micic, S.; Tepavcevic, D.K. Erectile dysfunction as a predictor of advanced vascular age. Andrology 2015, 3, 1125–1131. [Google Scholar] [CrossRef] [Green Version]
- Blumentals, W.A.; Gomez-Caminero, A.; Joo, S.; Vannappagari, V. Should erectile dysfunction be considered as a marker for acute myocardial infarction? Results from a retrospective cohort study. Int. J. Impot. Res. 2004, 16, 350–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolosi, A.; Glasser, D.B.; Moreira, E.D.; Villa, M. Prevalence of erectile dysfunction and associated factors among men without concomitant diseases: A population study. Int. J. Impot. Res. 2003, 15, 253–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Kang, J.; Li, Z.; Wang, X.; Liu, K.; Zhou, K.; Wang, W.; Shen, C. The association between plant-based diet and erectile dysfunction in Chinese men. Basic Clin. Androl. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, F.; De Sio, M.; Carleo, D.; Di Palo, C.; D’Armiento, M. Dietary factors in erectile dysfunction. Int. J. Impot. Res. 2006, 18, 370–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giugliano, F.; Maiorino, M.I.; Bellastella, G.; Autorino, R.; De Sio, M.; Giugliano, D.; Esposito, K. Adherence to mediterranean diet and erectile dysfunction in men with type 2 diabetes. J. Sex. Med. 2010, 7, 1911–1917. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; Hu, F.B. Nutrition and the endothelium. Curr. Diab. Rep. 2004, 4, 253–259. [Google Scholar] [CrossRef]
- Trebaticky, B.; Muchova, J.; Ziaran, S.; Bujdak, P.; Breza, J.; Durackova, Z. Natural polyphenols improve erectile function and lipid profile in patients suffering from erectile dysfunction. Bratisl. Med. J. 2019, 120, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Huetos, A.S.; Muralidharan, J.; Galiè, S.; Salas-Salvadó, J.; Bulló, M. Effect of nut consumption on erectile and sexual function in healthy males: A Secondary outcome analysis of the fertinuts randomized controlled trial. Nutrients 2019, 11, 1372. [Google Scholar] [CrossRef] [Green Version]
- Mykoniatis, I.; Grammatikopoulou, M.G.; Bouras, E.; Karampasi, E.; Tsionga, A.; Kogias, A.; Vakalopoulos, I.; Haidich, A.-B.; Chourdakis, M. Sexual Dysfunction Among Young Men: Overview of Dietary Components Associated With Erectile Dysfunction. J. Sex. Med. 2018, 15, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; Franz, M.; Rimm, E.B. Dietary flavonoid intake and incidence of erectile dysfunction. Am. J. Clin. Nutr. 2016, 103, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Bauer, S.R.; Breyer, B.N.; Stampfer, M.J.; Rimm, E.B.; Giovannucci, E.L.; Kenfield, S.A. Association of Diet with Erectile Dysfunction among Men in the Health Professionals Follow-up Study. JAMA Netw. Open 2020, 3, e2021701. [Google Scholar] [CrossRef]
- McNeal, J.E. Normal Histology of the Prostate. Am. J. Surg. Pathol. 1988, 12, 619–633. [Google Scholar] [CrossRef]
- Broggi, G.; Lo Giudice, A.; Di Mauro, M.; Asmundo, M.G.; Pricoco, E.; Piombino, E.; Caltabiano, R.; Morgia, G.; Russo, G.I. SRSF-1 and microvessel density immunohistochemical analysis by semi-automated tissue microarray in prostate cancer patients with diabetes (DIAMOND study). Prostate 2021, 81, 882–892. [Google Scholar] [CrossRef]
- Broggi, G.; Lo Giudice, A.; Di Mauro, M.; Pricoco, E.; Piombino, E.; Ferro, M.; Caltabiano, R.; Morgia, G.; Russo, G.I. Insulin signaling, androgen receptor and PSMA immunohistochemical analysis by semi-automated tissue microarray in prostate cancer with diabetes (DIAMOND study). Transl. Res. 2021, 238, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Adaramoye, O.A.; Akanni, O.O.; Abiola, O.J.; Owumi, S.E.; Akinloye, O.; Olapade-olaopa, E.O. Methyl jasmonate reduces testosterone-induced benign prostatic hyperplasia through regulation of inflammatory and apoptotic processes in rats. Biomed. Pharmacother. 2017, 95, 1493–1503. [Google Scholar] [CrossRef]
- Quintero-García, M.; Delgado-González, E.; Sánchez-Tusie, A.; Vázquez, M.; Aceves, C.; Anguiano, B. Iodine prevents the increase of testosterone-induced oxidative stress in a model of rat prostatic hyperplasia. Free Radic. Biol. Med. 2018, 115, 298–308. [Google Scholar] [CrossRef]
- Scott Lucia, M.; Lambert, J.R. Growth factors in benign prostatic hyperplasia: Basic science implications. Curr. Urol. Rep. 2008, 9, 272–278. [Google Scholar] [CrossRef]
- Shankar, E.; Bhaskaran, N.; MacLennan, G.T.; Liu, G.; Daneshgari, F.; Gupta, S. Inflammatory Signaling Involved in High-Fat Diet Induced Prostate Diseases. J. Urol. Res. 2015, 2, 1018. [Google Scholar]
- Denis, L.; Morton, M.S.; Griffiths, K. Diet and Its Preventive Role in Prostatic Disease. Eur. Urol. 1999, 35, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Penson, D.F.; Albertsen, P.C. Lessons learnt about early prostate cancer from large scale databases: Population-based pearls of wisdom. Surg. Oncol. 2002, 11, 3–11. [Google Scholar] [CrossRef]
- Sebastiano, C. Dietary patterns and prostatic diseases. Front. Biosci. 2012, E4, 195. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Luo, B.; Xu, S.; Fu, L.; Chen, Y.-H.; Zhang, C.; Wang, H.; Xie, D.-D.; Xu, D.-X. Vitamin D deficiency promotes prostatic hyperplasia in middle-age mice through exacerbating local inflammation. J. Steroid Biochem. Mol. Biol. 2018, 182, 14–20. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Shi, B.-K.; Li, J.-Y.; Zhu, X.-W.; Liu, J.; Liu, Y.-L. Role of p-ERK1/2 in Benign Prostatic Hyperplasia during Hyperinsulinemia. Urol. J. 2020, 18, 225–229. [Google Scholar] [CrossRef]
- de Amorim Ribeiro, I.C.; da Costa, C.A.S.; da Silva, V.A.P.; Côrrea, L.B.N.S.; Boaventura, G.T.; Chagas, M.A. Flaxseed reduces epithelial proliferation but does not affect basal cells in induced benign prostatic hyperplasia in rats. Eur. J. Nutr. 2017, 56, 1201–1210. [Google Scholar] [CrossRef]
- Said, M.M.; Hassan, N.S.; Schlicht, M.J.; Bosland, M.C. Flaxseed Suppressed Prostatic Epithelial Proliferation in a Rat Model of Benign Prostatic Hyperplasia. J. Toxicol. Environ. Health Part A 2015, 78, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Kayode, O.T.; Owolabi, A.V.; Kayode, A.A.A. Biochemical and histomorphological changes in testosterone propionate-induced benign prostatic hyperplasia in male Wistar rats treated with ketogenic diet. Biomed. Pharmacother. 2020, 132, 110863. [Google Scholar] [CrossRef]
- Chen, J.; Song, H. Protective potential of epigallocatechin-3-gallate against benign prostatic hyperplasia in metabolic syndrome rats. Environ. Toxicol. Pharmacol. 2016, 45, 315–320. [Google Scholar] [CrossRef]
- Aljehani, A.A.; Albadr, N.A.; Nasrullah, M.Z.; Neamatallah, T.; Eid, B.G.; Abdel-Naim, A.B. Icariin ameliorates metabolic syndrome-induced benign prostatic hyperplasia in rats. Environ. Sci. Pollut. Res. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-H.; Chen, C.-W.; Yu, P.-L.; Lin, Y.-L.; Hsieh, R-H. Mangosteen pericarp components alleviate progression of prostatic hyperplasia and mitochondrial dysfunction in rats. Sci. Rep. 2020, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.J.; Coffey, D.S.; Walsh, P.C.; Ewing, L.L. The Development of Human Benign Prostatic Hyperplasia with Age. J. Urol. 1984, 132, 474–479. [Google Scholar] [CrossRef]
- Chughtai, B.; Forde, J.C.; Thomas, D.D.M.; Laor, L.; Hossack, T.; Woo, H.H.; Te, A.E.; Kaplan, S.A. Benign prostatic hyperplasia. Nat. Rev. Dis. Prim. 2016, 2, 16031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, J.K. Modifiable Risk Factors for Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms: New Approaches to Old Problems. J. Urol. 2007, 178, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Pashootan, P.; Ploussard, G.; Cocaul, A.; De Gouvello, A.; Desgrandchamps, F. Association between metabolic syndrome and severity of lower urinary tract symptoms (LUTS): An observational study in a 4666 European men cohort. BJU Int. 2015, 116, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristal, A.R.; Arnold, K.B.; Schenk, J.M.; Neuhouser, M.L.; Goodman, P.; Penson, D.F.; Thompson, I.M. Dietary Patterns, Supplement Use, and the Risk of Symptomatic Benign Prostatic Hyperplasia: Results from the Prostate Cancer Prevention Trial. Am. J. Epidemiol. 2008, 167, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Obermuller-Jevic, U.C.; Hellmis, E.; Koch, W.; Jacobi, G.; Biesalski, H.-K. Lycopene Inhibits Disease Progression in Patients with Benign Prostate Hyperplasia. J. Nutr. 2008, 138, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Bowen, P.; Chen, L.; Duncan, C.; Ghosh, L.; Sharifi, R.; Christov, K. Effects of Tomato Sauce Consumption on Apoptotic Cell Death in Prostate Benign Hyperplasia and Carcinoma. Nutr. Cancer 2003, 47, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Giovannucci, E.; Willett, W.C.; Platz, E.A. Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. Am. J. Clin. Nutr. 2007, 85, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Lagiou, P.; Wuu, J.; Trichopoulou, A.; Hsieh, C.-C.; Adami, H.-O.; Trichopoulos, D. Diet and benign prostatic hyperplasia: A study in Greece. Urology 1999, 54, 284–290. [Google Scholar] [CrossRef]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of Human Prostate Cancer by Oral Administration of Green Tea Catechins in Volunteers with High-Grade Prostate Intraepithelial Neoplasia: A Preliminary Report from a One-Year Proof-of-Principle Study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Platz, E.A.; Kawachi, I.; Willett, W.C.; Giovannucci, E. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am. J. Clin. Nutr. 2002, 75, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, X.; Liu, Y.; Tian, H.; Flickinger, B.; Empie, M.W.; Sun, S.Z. Effects of Dietary Flaxseed Lignan Extract on Symptoms of Benign Prostatic Hyperplasia. J. Med. Food 2008, 11, 207–214. [Google Scholar] [CrossRef]
- Vahlensieck, W.; Theurer, C.; Pfitzer, E.; Patz, B.; Banik, N.; Engelmann, U. Effects of Pumpkin Seed in Men with Lower Urinary Tract Symptoms due to Benign Prostatic Hyperplasia in the One-Year, Randomized, Placebo-Controlled GRANU Study. Urol. Int. 2015, 94, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.I.; Larganà, G.; Sebastianelli, A.; Cocci, A.; Mauro, M.; Di Rapallo, I.; Morgia, G.; Morgia, M.; La Vignera, S.; Condorelli, R.; et al. The Investigative Role of Statins in Ameliorating Lower Urinary Tract Symptoms (LUTS): A Systematic Review. J. Clin. Med. 2021, 10, 416. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, G.I.; Broggi, G.; Cocci, A.; Capogrosso, P.; Falcone, M.; Sokolakis, I.; Gül, M.; Caltabiano, R.; Di Mauro, M. Relationship between Dietary Patterns with Benign Prostatic Hyperplasia and Erectile Dysfunction: A Collaborative Review. Nutrients 2021, 13, 4148. https://doi.org/10.3390/nu13114148
Russo GI, Broggi G, Cocci A, Capogrosso P, Falcone M, Sokolakis I, Gül M, Caltabiano R, Di Mauro M. Relationship between Dietary Patterns with Benign Prostatic Hyperplasia and Erectile Dysfunction: A Collaborative Review. Nutrients. 2021; 13(11):4148. https://doi.org/10.3390/nu13114148
Chicago/Turabian StyleRusso, Giorgio Ivan, Giuseppe Broggi, Andrea Cocci, Paolo Capogrosso, Marco Falcone, Ioannis Sokolakis, Murat Gül, Rosario Caltabiano, and Marina Di Mauro. 2021. "Relationship between Dietary Patterns with Benign Prostatic Hyperplasia and Erectile Dysfunction: A Collaborative Review" Nutrients 13, no. 11: 4148. https://doi.org/10.3390/nu13114148