Intravenous Lipid Emulsions Affect Respiratory Outcome in Preterm Newborn: A Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Nutritional Protocol
2.4. Statistics
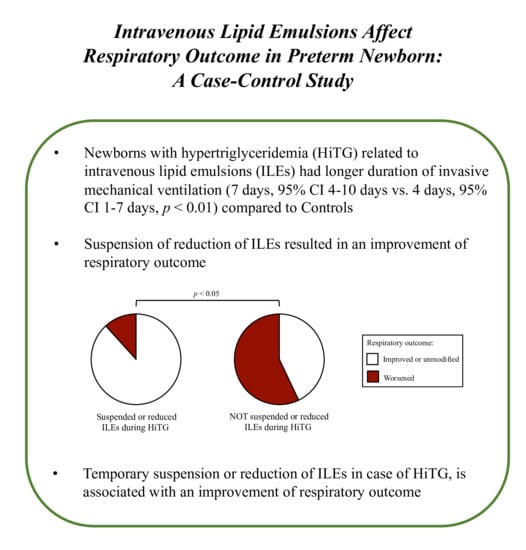
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Energy. Clin. Nutr. 2018, 37, 2309–2314. [Google Scholar] [CrossRef]
- Lapillonne, A.; Fidler Mis, N.; Goulet, O.; van den Akker, C.H.P.; Wu, J.; Koletzko, B.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; et al. ESPGHAN/ESPEN/ESPR/CSPEN Guidelines on Pediatric Parenteral Nutrition: Lipids. Clin. Nutr. 2018, 37, 2324–2336. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Coscia, A.; Boscarino, G.; Faccioli, F.; Di Chiara, M.; Greco, C.; Onestà, E.; Oliva, S.; Aloi, M.; Dito, L.; et al. Long-Term Effects on Growth of an Energy-Enhanced Parenteral Nutrition in Preterm Newborn: A Quasi-Experimental Study. PLoS ONE 2020, 15, e0235540. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, H.J.; Strommen, K.; Lang, A.M.; Abrahamsen, T.G.; Steen, E.K.; Pripp, A.H.; Ronnestad, A.E. Early Enhanced Parenteral Nutrition, Hyperglycemia, and Death Among Extremely Low-Birth-Weight Infants. JAMA Pediatr. 2015, 169, 1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrin, G.; Boscarino, G.; Gasparini, C.; Chiara, M.D.; Faccioli, F.; Onestà, E.; Parisi, P.; Spalice, A.; De Nardo, M.C.; Dito, L.; et al. Energy-Enhanced Parenteral Nutrition and Neurodevelopment of Preterm Newborns: A Cohort Study. Nutrition 2021, 111219. [Google Scholar] [CrossRef]
- Bonsante, F.; Gouyon, J.-B.; Robillard, P.-Y.; Gouyon, B.; Iacobelli, S. Early Optimal Parenteral Nutrition and Metabolic Acidosis in Very Preterm Infants. PLoS ONE 2017, 12, e0186936. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Goulet, O.; Hunt, J.; Krohn, K.; Shamir, R.; Parenteral Nutrition Guidelines Working Group; European Society for Clinical Nutrition and Metabolism; European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN); European Society of Paediatric Research (ESPR) 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR). J. Pediatr. Gastroenterol. Nutr. 2005, 41 Suppl 2, S1–S87. [Google Scholar] [CrossRef] [Green Version]
- Prasertsom, W.; Phillipos, E.Z.; Van Aerde, J.E.; Robertson, M. Pulmonary Vascular Resistance during Lipid Infusion in Neonates. Arch. Dis. Child. Fetal Neonatal Ed. 1996, 74, F95–F98. [Google Scholar] [CrossRef] [Green Version]
- Puntis, J.W.; Rushton, D.I. Pulmonary Intravascular Lipid in Neonatal Necropsy Specimens. Arch. Dis. Child. 1991, 66, 26–28. [Google Scholar] [CrossRef]
- Brans, Y.W.; Dutton, E.B.; Andrew, D.S.; Menchaca, E.M.; West, D.L. Fat Emulsion Tolerance in Very Low Birth Weight Neonates: Effect on Diffusion of Oxygen in the Lungs and on Blood PH. Pediatrics 1986, 78, 79–84. [Google Scholar] [PubMed]
- Passariello, A. Diarrhea in Neonatal Intensive Care Unit. World J. Gastroenterol. 2010, 16, 2664. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Terrin, G. Recent Progress in Congenital Diarrheal Disorders. Curr. Gastroenterol. Rep. 2011, 13, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; van Karnebeek, C.D.M. Inborn errors of metabolism. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 162, pp. 449–481. ISBN 978-0-444-64029-1. [Google Scholar]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano, F.; et al. Cow’s Milk and Rice Fermented with Lactobacillus Paracasei CBA L74 Prevent Infectious Diseases in Children: A Randomized Controlled Trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Salvia, G.; Cascioli, C.F.; Ciccimarra, F.; Terrin, G.; Cucchiara, S. A Case of Protein-Losing Enteropathy Caused by Intestinal Lymphangiectasia in a Preterm Infant. Pediatrics 2001, 107, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Passariello, A.; Terrin, G.; Cecere, G.; Micillo, M.; Marco, G.; Di Costanzo, M.; Cosenza, L.; Leone, L.; Nocerino, R.; Berni Canani, R. Randomised Clinical Trial: Efficacy of a New Synbiotic Formulation Containing Lactobacillus Paracasei B21060 plus Arabinogalactan and Xilooligosaccharides in Children with Acute Diarrhoea. Aliment. Pharmacol. Ther. 2012, 35, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Di Chiara, M.; Boscarino, G.; Versacci, P.; Di Donato, V.; Giancotti, A.; Pacelli, E.; Faccioli, F.; Onestà, E.; Corso, C.; et al. Echocardiography-Guided Management of Preterms With Patent Ductus Arteriosus Influences the Outcome: A Cohort Study. Front. Pediatr. 2020, 8, 582735. [Google Scholar] [CrossRef]
- Terrin, G.; Di Chiara, M.; Boscarino, G.; Metrangolo, V.; Faccioli, F.; Onestà, E.; Giancotti, A.; Di Donato, V.; Cardilli, V.; De Curtis, M. Morbidity Associated with Patent Ductus Arteriosus in Preterm Newborns: A Retrospective Case-Control Study. Ital. J. Pediatr. 2021, 47, 9. [Google Scholar] [CrossRef]
- Peila, C.; Spada, E.; Giuliani, F.; Maiocco, G.; Raia, M.; Cresi, F.; Bertino, E.; Coscia, A. Extrauterine Growth Restriction: Definitions and Predictability of Outcomes in a Cohort of Very Low Birth Weight Infants or Preterm Neonates. Nutrients 2020, 12, 1224. [Google Scholar] [CrossRef]
- Terrin, G.; Boscarino, G.; Di Chiara, M.; Iacobelli, S.; Faccioli, F.; Greco, C.; Onestà, E.; Sabatini, G.; Pietravalle, A.; Oliva, S.; et al. Nutritional Intake Influences Zinc Levels in Preterm Newborns: An Observational Study. Nutrients 2020, 12, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrin, G.; Passariello, A.; Canani, R.B.; Manguso, F.; Paludetto, R.; Cascioli, C. Minimal Enteral Feeding Reduces the Risk of Sepsis in Feed-Intolerant Very Low Birth Weight Newborns. Acta Paediatr. 2009, 98, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Passariello, A.; Buccigrossi, V.; Terrin, G.; Guarino, A. The Nutritional Modulation of the Evolving Intestine. J. Clin. Gastroenterol. 2008, 42, S197–S200. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; De Nardo, M.C.; Boscarino, G.; Di Chiara, M.; Cellitti, R.; Ciccarelli, S.; Gasparini, C.; Parisi, P.; Urna, M.; Ronchi, B.; et al. Early Protein Intake Influences Neonatal Brain Measurements in Preterms: An Observational Study. Front. Neurol. 2020, 11, 885. [Google Scholar] [CrossRef] [PubMed]
- Lekka, M.E.; Liokatis, S.; Nathanail, C.; Galani, V.; Nakos, G. The Impact of Intravenous Fat Emulsion Administration in Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2004, 169, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Hageman, J.R.; Hunt, C.E. Fat Emulsions and Lung Function. Clin. Chest Med. 1986, 7, 69–77. [Google Scholar] [CrossRef]
- Suchner, U.; Katz, D.P.; Fürst, P.; Beck, K.; Felbinger, T.W.; Senftleben, U.; Thiel, M.; Goetz, A.E.; Peter, K. Effects of Intravenous Fat Emulsions on Lung Function in Patients with Acute Respiratory Distress Syndrome or Sepsis. Crit. Care Med. 2001, 29, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Periera, G.R.; Fox, W.W.; Stanley, C.A.; Baker, L.; Schwartz, J.G. Decreased Oxygenation and Hyperlipemia during Intravenous Fat Infusions in Premature Infants. Pediatrics 1980, 66, 26–30. [Google Scholar] [PubMed]
- Giretti, I.; D’Ascenzo, R.; Correani, A.; Antognoli, L.; Monachesi, C.; Biagetti, C.; Pompilio, A.; Marinelli, L.; Burattini, I.; Cogo, P.; et al. Hypertriglyceridemia and Lipid Tolerance in Preterm Infants with a Birth Weight of Less than 1250 g on Routine Parenteral Nutrition. Clin. Nutr. Edinb. Scotl. 2021. [Google Scholar] [CrossRef] [PubMed]
- Correani, A.; Giretti, I.; Antognoli, L.; Monachesi, C.; Cogo, P.; D’Ascenzo, R.; Biagetti, C.; Carnielli, V.P. Hypertriglyceridemia and Intravenous Lipid Titration During Routine Parenteral Nutrition in Small Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 619–625. [Google Scholar] [CrossRef]
- Sinclair, R.; Schindler, T.; Lui, K.; Bolisetty, S. Hypertriglyceridaemia in Extremely Preterm Infants Receiving Parenteral Lipid Emulsions. BMC Pediatr. 2018, 18, 348. [Google Scholar] [CrossRef] [PubMed]
- Holtrop, P.; Swails, T.; Riggs, T. Hypertriglyceridemia in Extremely Low Birth Weight Infants Receiving Lipid Emulsions. J. Neonatal-Perinat. Med. 2015, 8, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Toce, S.S.; Keenan, W.J. Lipid Intolerance in Newborns Is Associated with Hepatic Dysfunction but Not Infection. Arch. Pediatr. Adolesc. Med. 1995, 149, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Vlaardingerbroek, H.; van Goudoever, J.B. Intravenous Lipids in Preterm Infants: Impact on Laboratory and Clinical Outcomes and Long-Term Consequences. In World Review of Nutrition and Dietetics; Calder, P.C., Waitzberg, D.L., Koletzko, B., Eds.; S. KARGER AG: Basel, Switzerland, 2014; Volume 112, pp. 71–80. ISBN 978-3-318-02752-5. [Google Scholar]
- Pereira-da-Silva, L.; Nóbrega, S.; Rosa, M.L.; Alves, M.; Pita, A.; Virella, D.; Papoila, A.L.; Serelha, M.; Cordeiro-Ferreira, G.; Koletzko, B. Parenteral Nutrition-Associated Cholestasis and Triglyceridemia in Surgical Term and near-Term Neonates: A Pilot Randomized Controlled Trial of Two Mixed Intravenous Lipid Emulsions. Clin. Nutr. ESPEN 2017, 22, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-C.; Lin, H.-C.; Chang, Y.-J.; Yang, S.-P.; Tsao, L.-Y.; Lee, C.-H.; Chen, H.-N.; Chen, J.-Y.; Tsai, Y.-G. Intravenous Fish Oil Containing Lipid Emulsion Attenuates Inflammatory Cytokines and the Development of Bronchopulmonary Dysplasia in Very Premature Infants: A Double-Blind, Randomized Controlled Trial. Clin. Nutr. 2019, 38, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, C.; Johnson, K.; Miall, L.S.; Thompson, D.; Puntis, J. The Effect of Parenteral Lipid Emulsions on Pulmonary Hemodynamics and Eicosanoid Metabolites in Preterm Infants: A Pilot Study. Nutr. Clin. Pract. 2013, 28, 753–757. [Google Scholar] [CrossRef] [PubMed]


| Cases of HiTG (n = 40) | Controls (n = 105) | OR (95% CI) | p Value | |
|---|---|---|---|---|
| Maternal age, years | 34 (29 to 38) | 37 (34 to 40) | - | 0.385 |
| ≥35 years, No. (%) | 20 (50.0) | 52 (49.5) | 1.291 (0.587 to 2841) | 0.525 |
| Gestational age, weeks | 26 (25 to 28) | 28 (27 to 29) | - | <0.001 |
| ≤28 + 6/7 weeks, No. (%) | 28 (70.0) | 51 (48.6) | 0.371 (0.167 to 0.822) | 0.013 |
| Birth weight, g | 807 (633 to 981) | 1066 (906 to 1226) | - | <0.001 |
| ELBW, No. (%) | 27 (67.5) | 33 (31.4) | 4909 (2217 to 10.872) | <0.001 |
| SGA, No. (%) | 12 (30.0) | 17 (16.2) | 2.701 (1132 to 6446) | 0.022 |
| Male sex, No. (%) | 16 (40.0) | 50 (47.6) | 0.733 (0.350 to 1536) | 0.410 |
| Cesarean section, No. (%) | 29 (72.5) | 94 (89.5) | 0.485 (0.172 to 1365) | 0.136 |
| Prenatal steroids administration a, No. (%) | 19 (47.5) | 71 (67.6) | 0.535 (0.247 to 1158) | 0.110 |
| Intrauterine growth restriction, No (%) | 5 (12.5) | 8 (7.6) | 1956 (0.596 to 6417) | 0.210 |
| Gestational diabetes, No (%) | 1 (2.5) | 10 (9.5) | 0.266 (0.033 to 2153) | 0.168 |
| Abruptio placentae, No (%) | 4 (10.0) | 10 (9.5) | 1188 (0.348 to 4050) | 0.501 |
| Pregnancy-induced hypertension, No. (%) | 11 (27.5) | 26 (24.5) | 1337 (0.579 to 3085) | 0.495 |
| Thyroid dysfunction, No. (%) | 6 (15.0) | 11 (10.5) | 1.709 (0.583 to 5014) | 0.240 |
| Twins, No. (%) | 10 (25.0) | 36 (34.3) | 0.737 (0.320 to 1696) | 0.472 |
| 5-min Apgar score | 6 (5 to 7) | 7 (6 to 8) | - | 0.078 |
| <5, No. (%) | 7 (17.5) | 5 (4.8) | 0.207 (0.061 to 0.702) | 0.012 |
| pH at birth | 7.2 (7.1 to 7.3) | 7.2 (7.1 to 7.3) | - | 0.058 |
| ≤7.1, No. (%) | 1 (2.5) | 4 (3.8) | 1414 (0.153 to 13.085) | 0.614 |
| Birth weight gain before 14 DOL, No. (%) | 23 (57.5) | 79 (75.2) | 0.556 (0.234 to 1320) | 0.180 |
| Overall | GA ≤ 28 + 6/7 Weeks or ELBW | |||
|---|---|---|---|---|
| Cases of HiTG (n = 40) | Controls (n = 105) | Cases of HiTG (n = 34) | Controls (n = 57) | |
| Bronchopulmonary Dysplasia | 12 (30.0) * | 15 (14.3) | 12 (35.3) | 13 (22.8) |
| Caffeine administration | 35 (87.5) | 97 (92.4) | 29 (85.3) | 56 (98.2) |
| Postnatal steroids administration | 13 (32.5) ** | 14 (13.3) | 13 (38.2) * | 12 (21.1) |
| Invasive mechanical ventilation | 23 (57.5) ** | 34 (32.4) | 22 (64.7) * | 26 (45.6) |
| Duration, mean days (95% CI) | 7 (4 to 10) ** | 4 (1 to 7) | 7 (4 to 10) * | 5 (1 to 8) |
| ≥7 days | 10 (25.0) ** | 6 (5.7) | 10 (29.4) ** | 6 (10.5) |
| Noninvasive mechanical ventilation | 34 (85.0) | 89 (84.8) | 29 (85.3) | 49 (86.0) |
| Duration, mean days (95% CI) | 16 (8 to 23) | 16 (10 to 21) | 16 (8 to 24) | 18 (12 to 25) |
| ≥7 days | 16 (40.0) | 29 (27.6) | 16 (47.1) | 26 (45.6) |
| Covariates | Overall | GA ≤ 28 +6/7 Weeks or ELBW |
|---|---|---|
| FEF ≥ 15 days (not or yes) | 4106 (0.806 to 20.931) | 3746 (0.709 to 19.784) |
| Hypertriglyceridemia (not or yes) | 6219 (1655 to 23.371) ** | 5420 (1395 to 21.049) * |
| Prenatal steroids administration a (not or yes) | 1821 (0.496 to 6690) | 1252 (0.332 to 4722) |
| Surfactant administration (not or yes) | 2592 (0.629 to 10.686) | 1799 (0.407 to 7959) |
| SGA at birth (not or yes) | 0.510 (0.105 to 2487) | 0.483 (0.095 to 2454) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscarino, G.; Conti, M.G.; De Luca, F.; Di Chiara, M.; Deli, G.; Bianchi, M.; Favata, P.; Cardilli, V.; Di Nardo, G.; Parisi, P.; et al. Intravenous Lipid Emulsions Affect Respiratory Outcome in Preterm Newborn: A Case-Control Study. Nutrients 2021, 13, 1243. https://doi.org/10.3390/nu13041243
Boscarino G, Conti MG, De Luca F, Di Chiara M, Deli G, Bianchi M, Favata P, Cardilli V, Di Nardo G, Parisi P, et al. Intravenous Lipid Emulsions Affect Respiratory Outcome in Preterm Newborn: A Case-Control Study. Nutrients. 2021; 13(4):1243. https://doi.org/10.3390/nu13041243
Chicago/Turabian StyleBoscarino, Giovanni, Maria Giulia Conti, Francesca De Luca, Maria Di Chiara, Giorgia Deli, Marco Bianchi, Paola Favata, Viviana Cardilli, Giovanni Di Nardo, Pasquale Parisi, and et al. 2021. "Intravenous Lipid Emulsions Affect Respiratory Outcome in Preterm Newborn: A Case-Control Study" Nutrients 13, no. 4: 1243. https://doi.org/10.3390/nu13041243