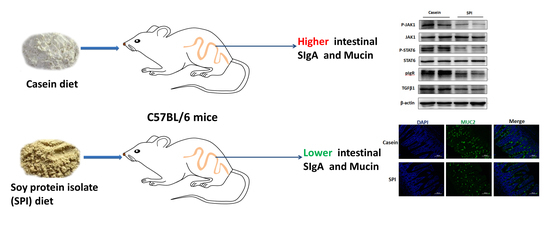
Dietary Soy Protein Isolate Attenuates Intestinal Immunoglobulin and Mucin Expression in Young Mice Compared with Casein
Abstract
:1. Introduction
2. Materials and Method
2.1. Diets
2.2. Animal Experiments
2.3. Enzyme-Linked Immunosorbent Assay
2.4. Gene Expression Analysis Using qRT-PCR
2.5. Western Blotting Analysis
2.6. Periodic Acid-Schiff Staining
2.7. Immunofluorescence
2.8. Statistical Analysis
3. Results
3.1. Body Weight and Feed Intake
3.2. SPI Reduces Intestinal Secretory IgA (SIgA) and Th2 Cytokine Levels
3.3. SPI Suppresses the Intestinal JAK1/STAT6 Signaling Pathway
3.4. SPI Reduces Intestinal Mucin Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PAS | Periodic Acid–Schiff |
| SPI | Soy protein isolate |
| SIgA | Secretory immunoglobulin A |
| IL-1β | Interleukin-1β |
| IL-4 | Interleukin-4 |
| IL-5 | Interleukin-5 |
| IL-13 | Interleukin-13 |
| pIgR | Polymeric immunoglobulin receptor |
| Jak1 | Janus kinase 1 |
| Stat6 | Signal transducer and activator of transcription 6 |
| TGFβ | Transforming growth factor-β |
| Muc1 | Mucin 1 |
| Muc2 | Mucin 2 |
| Tff3 | Trefoil factor 3 |
| Grp94 | Glucose-regulated protein 94 |
| Agr2 | Anterior gradient homolog 2 |
References
- Roux, L.L.; Mejean, S.; Chacon, R.; Lopez, C.; Dupont, D.; Deglaire, A.; Nau, F.; Jeantet, R. Plant proteins partially replacing dairy proteins greatly influence infant formula functionalities. LWT Food Sci. Technol. 2020, 120, 108891. [Google Scholar] [CrossRef]
- Li, H.; Zhu, K.; Zhou, H.; Peng, W.; Guo, X. Comparative Study about Some Physical Properties, In vitro Digestibility and Immunoreactivity of Soybean Protein Isolate for Infant Formula. Plant Foods Hum. Nutr. 2013, 68, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, J.; Greer, F.R. Use of Soy Protein-Based Formulas in Infant Feeding. Pediatrics 2008, 121, 1062–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, M.A.T.; Mcneil, D.A.; Maloff, B.; Mutasingwa, D.; Wu, M.; Ford, C.; Tough, S. Reducing obesity and related chronic disease risk in children and youth: A synthesis of evidence with ‘best practice’ recommendations. Obes. Rev. 2006, 7, 7–66. [Google Scholar] [CrossRef]
- Uddin, M.N.; KanikaMitra, D.; Rahman, M.M.; Abdullah, A.; Haque, D.M.Z. Evaluation of proximate, determination of minerals and chromatographic quantification of water soluble vitamin in newly developed soy protein isolate. J. Biosci. 2016, 4, 604–608. [Google Scholar]
- Ronis, M.J.; Chen, Y.; Badeaux, J.; Badger, T.M. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J. Nutr. 2009, 139, 1431–1438. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, T.B. Soy, soy phytoestrogens and cardiovascular disease. J. Nutr. 2002, 132, 566S–569S. [Google Scholar] [CrossRef]
- Badger, T.M.; Ronis, M.J.J.; Hakkak, R.; Rowlands, J.C.; Korourian, S. The Health Consequences of Early Soy Consumption. J. Nutr. 2002, 132, 559–565. [Google Scholar] [CrossRef]
- Damiano, S.; Sasso, A.; De Felice, B.; Di Gregorio, I.; La Rosa, G.; Lupoli, G.A.; Belfiore, A.; Mondola, P.; Santillo, M. Quercetin increases MUC2 and MUC5AC gene expression and secretion in intestinal goblet cell-like LS174T via PLC/PKCα/ERK1-2 pathway. Front. Physiol. 2018, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Tian, Y.; Wu, Y.; Ma, X. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr. Protein Pept. Sci. 2017, 18, 795–808. [Google Scholar] [CrossRef]
- Hukkinen, M.; Merrassalmio, L.; Pakarinen, M.P. Health-related quality of life and neurodevelopmental outcomes among children with intestinal failure. Semin. Pediatr. Surg. 2018, 27, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsayed, N.M.; Ramadan, M.E. The Impact of Intestinal Parasitic Infections on the Health Status of Children: An Overview. J. Pediatr. Infect. Dis. 2017, 12, 209–213. [Google Scholar]
- Ji, F.J.; Wang, L.X.; Yang, H.; Hu, A.; Yin, Y. Review: The roles and functions of glutamine on intestinal health and performance of weaning pigs. Animal 2019, 13, 2727–2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Bi, J.; Lei, Q.; Wan, X.; Jiang, T.; Wu, C.; Wang, X. Partial enteral nutrition increases intestinal sIgA levels in mice undergoing parenteral nutrition in a dose-dependent manner. Int. J. Surg. 2018, 49, 74–79. [Google Scholar] [CrossRef]
- Marchbank, T.; Mandir, N.; Calnan, D.; Goodlad, R.A.; Podas, T.; Playford, R.J. Specific protein supplementation using soya, casein or whey differentially affects regional gut growth and luminal growth factor bioactivity in rats; implications for the treatment of gut injury and stimulating repair. Food Funct. 2018, 9, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Huang, Z.; Zhou, G.; Li, H.; Xu, X.; Li, C. Dietary proteins rapidly altered the microbial composition in rat caecum. Curr. Microbiol. 2017, 74, 1447–1452. [Google Scholar] [CrossRef]
- Graf, D.; Di Cagno, R.; Fåk, F.; Flint, H.J.; Nyman, M.; Saarela, M.; Watzl, B. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 2015, 26, 26164. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Pabst, O. New concepts in the generation and functions of IgA. Nat. Reviews Immunol. 2012, 12, 821–832. [Google Scholar] [CrossRef]
- Heneghan, A.F.; Pierre, J.F.; Kudsk, K.A. JAK-STAT and intestinal mucosal immunology. Jak Stat 2013, 2, e25530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Hua, C.; Zhao, F.; Li, M.; Fu, Q.; Hooiveld, G.J.; Muller, M.; Li, C.; Zhou, G. Purified dietary red and white meat proteins show beneficial effects on growth and metabolism of young rats compared to casein and soy protein. J. Agric. Food Chem. 2018, 66, 9942–9951. [Google Scholar] [CrossRef] [PubMed]
- FAQ Expert Consultation. Dietary protein quality evaluation in human nutrition. FAO Food Nutr. Pap. 2013, 92, 1–66. [Google Scholar]
- Agostoni, C.; Agostoni, C.; Axelsson, I.; Goulet, O.; Koletzko, B.; Michaelsen, K.F.; Puntis, J.; Rieu, D.; Rigo, J.; Shamir, R.; et al. Soy protein infant formulae and follow-on formulae: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Hooiveld, G.J.; Li, M.; Zhao, F.; Zhang, W.; Xu, X.; Muller, M.; Li, C.; Zhou, G. Dietary soy and meat proteins induce distinct physiological and gene expression changes in rats. Sci. Rep. 2016, 6, 20036. [Google Scholar] [CrossRef] [Green Version]
- Wróblewska, B.; Juśkiewicz, J.; Kroplewski, B.; Jurgoński, A.; Wasilewska, E.; Złotkowska, D.; Markiewicz, L. The effects of whey and soy proteins on growth performance, gastrointestinal digestion, and selected physiological responses in rats. Food Funct. 2018, 9, 1500–1509. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.P.; Bhandari, B.; Cichero, J.; Prakash, S. Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study. Food Res. Int. 2015, 76, 348–358. [Google Scholar] [CrossRef] [Green Version]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, H.; Liu, G.; Chen, S.; Tan, B.; Ren, W.; Bazer, F.W.; Wu, G.; Yin, Y. Glutamine promotes intestinal SIgA secretion through intestinal microbiota and IL-13. Mol. Nutr. Food Res. 2016, 60, 1637–1648. [Google Scholar] [CrossRef]
- Heneghan, A.F.; Pierre, J.F.; Kudsk, K.A. IL-25 improves IgA levels during parenteral nutrition through the JAK-STAT pathway. Ann. Surg. 2013, 258, 1065–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavnezer, J.; Kang, J. The surprising discovery that TGFβ specifically induces the IgA class switch. J. Immunol. 2009, 182, 5–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ginkel, F.W.; Wahl, S.M.; Kearney, J.F.; Kweon, M.N.; Fujihashi, K.; Burrows, P.D.; Kiyono, H.; Mcghee, J.R. Partial IgA-deficiency with increased Th2-type cytokines in TGF-beta 1 knockout mice. J. Immunol. 1999, 163, 1951. [Google Scholar] [PubMed]
- Konstantinou, G.N.; Bencharitiwong, R.; Grishin, A.; Caubet, J.C.; Bardina, L.; Sicherer, S.H.; Sampson, H.A.; Nowak-Węgrzyn, A. The role of casein-specific IgA and TGF-β in children with food protein-induced enterocolitis syndrome to milk. Pediatr. Allergy Immunol. 2014, 25, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Przybyszewski, J.; Mitra, D.; Becker, C.; Brehm-Stecher, B.; Tentinger, A.; MacDonald, R.S. Soy protein diet, but not Lactobacillus rhamnosus GG, decreases mucin-1, trefoil factor-3, and tumor necrosis factor-α in colon of dextran sodium sulfate-treated C57BL/6 mice. J. Nutr. 2011, 141, 1239–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, K.; Ota, H.; Akamatsu, T.; Sugiyama, A.; Katsuyama, T. Histochemistry of the surface mucous gel layer of the human colon. Gut 1997, 40, 782–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Staron, M.; Hong, F.; Wu, B.X.; Sun, S.; Morales, C.; Crosson, C.E.; Tomlinson, S.; Kim, I.; Wu, D. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 6877–6882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-W.; Zhen, G.; Verhaeghe, C.; Nakagami, Y.; Nguyenvu, L.T.; Barczak, A.J.; Killeen, N.; Erle, D.J. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl. Acad. Sci. USA 2009, 106, 6950–6955. [Google Scholar] [CrossRef] [Green Version]
- Ronis, M.J.; Gomez-Acevedo, H.; Shankar, K.; Sharma, N.; Blackburn, M.; Singhal, R.; Mercer, K.E.; Badger, T.M. Soy protein isolate feeding does not result in reproductive toxicity in the pre-pubertal rat testis. Exp. Biol. Med. 2018, 243, 695–707. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [Green Version]
- Kalman, D.S. Amino Acid Composition of an Organic Brown Rice Protein Concentrate and Isolate Compared to Soy and Whey Concentrates and Isolates. Foods 2014, 3, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, T.; Yin, Y.; Zhang, C.-Y.; Zhang, Y.-L. Dietary microRNA-A Novel Functional Component of Food. Adv. Nutr. 2019, 10, 711–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Lei, L.; Ye, F.; Zhou, Y.; Chang, H.; Zhao, G. Nutritive implications of dietary microRNAs: Facts, controversies, and perspectives. Food Funct. 2019, 10, 3044–3056. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Wang, R.; Luo, J.-Y.; He, J.-J.; Ye, R.-S.; Xie, M.-Y.; Xi, Q.-Y.; Jiang, Q.-Y.; Sun, J.-J.; et al. Plant MIR156 regulates intestinal growth in mammals by targeting the Wnt/beta-catenin pathway. Am. J. Physiol. Cell Physiol. 2019, 317, C434–C448. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Weng, Z.; Sui, X.; Fang, Y.; Tang, X.; Shen, X. Soybean-derived miRNAs specifically inhibit proliferation and stimulate apoptosis of human colonic Caco-2 cancer cells but not normal mucosal cells in culture. Genomics 2020, 112, 2949–2958. [Google Scholar] [CrossRef]
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J. Dairy Sci. 2012, 95, 4831–4841. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Shimaoka, M.; Kiyono, H. MicroRNA-mediated dynamic control of mucosal immunity. Int. Immunol. 2017, 29, 157–163. [Google Scholar] [CrossRef]
- Martinez-Augustin, O.; Rivero-Gutierrez, B.; Mascaraque, C.; Sanchez de Medina, F. Food Derived Bioactive Peptides and Intestinal Barrier Function. Int. J. Mol. Sci. 2014, 15, 22857–22873. [Google Scholar] [CrossRef] [Green Version]




| Item (%) | Casein | SPI |
|---|---|---|
| Asp | 6.71 | 10.30 |
| Thr | 3.76 | 3.37 |
| Ser | 4.38 | 4.19 |
| Glu | 18.78 | 16.60 |
| Gly | 1.74 | 3.73 |
| Ala | 2.90 | 3.88 |
| Val | 5.65 | 4.31 |
| Met | 2.29 | 0.73 |
| Ile | 4.53 | 4.14 |
| Leu | 8.50 | 7.02 |
| Tyr | 4.74 | 3.26 |
| Phe | 4.59 | 4.64 |
| Lys | 6.77 | 5.66 |
| His | 2.37 | 2.29 |
| Arg | 3.01 | 6.68 |
| Pro | 8.90 | 4.57 |
| Trp | 1.10 | 1.12 |
| Ingredient (g/kg) | Casein Diet | SPI Diet |
|---|---|---|
| Cornstarch | 397.468 | 397.468 |
| Casein (≥85% protein) | 200.000 | 0 |
| Soy protein isolate * | 0 | 200.000 |
| Dextrinized cornstarch | 132.000 | 132.000 |
| Sucrose | 100.000 | 100.000 |
| Soybean oil (no additives) | 70.000 | 70.000 |
| Fiber | 50.000 | 50.000 |
| Mineral mix (AIN-93G-MX) | 35.000 | 35.000 |
| Vitamin mix (AIN-93-VX) | 10.000 | 10.000 |
| L-Cystine | 3.000 | 3.000 |
| Choline bitartrate (41.1% choline) | 2.500 | 2.500 |
| Tert-butylhydroquinone | 0.014 | 0.014 |
| Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-actin | TGCTGTCCCTGTATGCCTCT | CTTTGATGTCACGCACGATTT |
| J-chain | GAACTTTGTATACCATTTGTCAGACG | CTGGGTGGCAGTAACAACCT |
| pIgR | AGTAACCGAGGCCTGTCCTT | GTCACTCGGCAACTCAGGA |
| TGFβ1 | ATTCCTGGCGTTACCTTGG | AGCCCTGTATTCCGTCTCCT |
| TGFβ2 | ACATCCACACGCACACTCAT | AAGGGACGAGACGAGAAGGT |
| JAK1 | AGTGCAGTATCTCTCCTCTCTG | GATTCGGTTCGGAGCGTACC |
| STAT6 | ATCTTCAACGACAACAGCCTCA | GGAGAAGGCTAGTGACATATTG |
| IL-1β | TTGAAGTTGACGGACCCCA | CCACAGCCACAATGAGTGATAC |
| IL-4 | AACGAGGTCACAGGAGAAGG | TGGAAGCCCTACAGACAAGC |
| IL-5 | CCCTCATCCTCTTCGTTGC | ATCCTCCTGCGTCCATCTG |
| IL-13 | CTTGCTTGCCTTGGTGGTC | GGGAGTCTGGTCTTGTGTGA |
| MUC1 | CCTTCAGTGCCAAGTCAATAC | TCCCCAGAAAATCTCCGTT |
| MUC2 | ATGCCCACCTCCTCAAAGAC | GTAGTTTCCGTTGGAACAGTGAA |
| TFF3 | GCTAATGCTGTTGGTGGTCC | GGTTGTTACACTGCTCCGATG |
| GRP94 | GGTGTTGTGGATTCCGATG | GAAGTTTAGCAAGCCGTGTT |
| AGR2 | CTGTTGCTTGTCTTGGATCTGT | GGAGCCAAAAAGGACCCAAAG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, B.; Wang, D.; Wang, H.; Chen, T.; Luo, J.; Xi, Q.; Sun, J.; Zhang, Y. Dietary Soy Protein Isolate Attenuates Intestinal Immunoglobulin and Mucin Expression in Young Mice Compared with Casein. Nutrients 2020, 12, 2739. https://doi.org/10.3390/nu12092739
Zeng B, Wang D, Wang H, Chen T, Luo J, Xi Q, Sun J, Zhang Y. Dietary Soy Protein Isolate Attenuates Intestinal Immunoglobulin and Mucin Expression in Young Mice Compared with Casein. Nutrients. 2020; 12(9):2739. https://doi.org/10.3390/nu12092739
Chicago/Turabian StyleZeng, Bin, Dongyang Wang, Hailong Wang, Ting Chen, Junyi Luo, Qianyun Xi, Jiajie Sun, and Yongliang Zhang. 2020. "Dietary Soy Protein Isolate Attenuates Intestinal Immunoglobulin and Mucin Expression in Young Mice Compared with Casein" Nutrients 12, no. 9: 2739. https://doi.org/10.3390/nu12092739