Constructing a Z-scheme Heterojunction of Egg-Like Core@shell CdS@TiO2 Photocatalyst via a Facile Reflux Method for Enhanced Photocatalytic Performance
Abstract
:1. Introduction
2. Materials and Methods
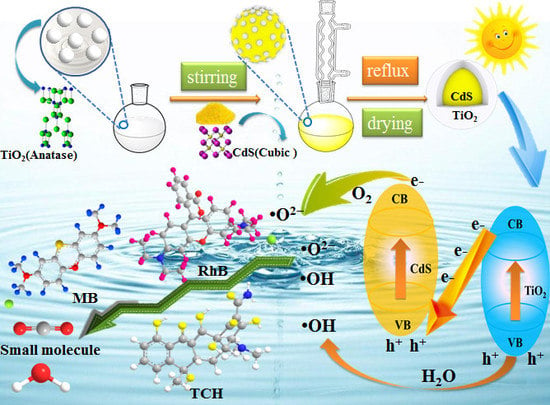
Preparation of Core-Shell Structure CdS@TiO2Composites
3. Results and Discussion
3.1. Fabrication of Core-Shell Structure
3.2. Photoelectrochemical Propertiesof Core-Shell Structure CdS@TiO2Photocatalyst
3.3. Photocatalystic Activity of Core-Shell Structure CdS@TiO2 Photocatalyst
3.4. Effects of Different Factors on Photodegradation
3.5. Mechanism of Pollutant Photodegradation
3.6. The Structure-Effective Relationship of Core-Shell Photocatalyst
3.7. Photostability over 0.10CdS@TiO2 under Visible Light
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Wu, Z.; Liu, D.; Gao, Z. Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res. Lett. 2016, 12, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Z.; Gao, Z.; Ye, B.C. Effect of different activated carbon as carrier on the photocatalytic activity of Ag-N-ZnO photocatalyst for methyl orange degradation under visible light irradiation. Nanomaterials 2017, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Gong, Y.; Hu, Z.; Li, Z.; Qiu, Z.; Zhu, X.; Fang, Z. Plasmonic hot electron enhanced MoS2 photocatalysis in hydrogen evolution. Nanoscale 2015, 7, 4482–4488. [Google Scholar] [CrossRef]
- Politano, A.; Cupolillo, A.; Di Profio, G.; Arafat, H.A.; Chiarello, G.; Curcio, E. When plasmonics meets membrane technology. J. Phys. Condens. Matter 2016, 28, 363003. [Google Scholar] [CrossRef] [PubMed]
- Michael, A. A surface science perspective on photocatalysis. Surf. Sci. Rep. 2011, 66, 6–7. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238(5358), 37–38. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wu, Z.; Yan, Y.; Ge, X.; Tong, Y. Photodegradation of formaldehyde by activated carbon loading TiO2 synthesized via microwave irradiation. Korean J. Chem. Eng. 2015, 32, 1333–1339. [Google Scholar]
- Chen, X.; Liu, D.; Wu, Z.; Cravotto, G.; Wu, Z.; Ye, B.C. Microwave-assisted rapid synthesis of Ag-β-cyclodextrin/TiO2/AC with exposed {001} facets for highly efficient naphthalene degradation under visible light. Catal. Commun. 2018, 104, 96–100. [Google Scholar] [CrossRef]
- Dong, W.; Pan, F.; Xu, L.; Zheng, M.; Sow, C.H.; Wu, K.; Xu, G.Q.; Chen, W. Facile synthesis of CdS@TiO2 core–shell nanorods with controllable shell thickness and enhanced photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 2015, 349, 279–286. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Wen, T.; Ren, X.; Huang, Y.; Wang, X. Adsorption of naphthalene and its derivatives on magnetic graphene composites and the mechanism investigation. Colloids Surf. A 2013, 422, 118–125. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Z.; Tian, F.; Ye, B.C.; Tong, Y. Synthesis of N and La co-doped TiO2/AC photocatalyst by microwave irradiation for the photocatalytic degradation of naphthalene. J. Alloy. Compd. 2016, 676, 489–498. [Google Scholar] [CrossRef]
- Tian, F.; Wu, Z.; Tong, Y.; Wu, Z.; Cravotto, G. Microwave-assisted synthesis of carbon-based (N, Fe)-codoped TiO2 for the photocatalytic degradation of formaldehyde. Nanoscale Res. Lett. 2015, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Bera, R.; Kundu, S.; Patra, A. 2D hybrid nanostructure of reduced graphene oxide–CdS nanosheet for enhanced photocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 13251–13259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, W.; Chen, X.; Wang, J.; Zhu, Y. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal. B 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.W.; Zou, Y.; Fan, Z.; Ji, X.; Niu, C.; Wang, L. Rational design of CdS@ZnO core-shell structure via atomic layer deposition for drastically enhanced photocatalytic H2 evolution with excellent photostability. Nano Energy 2017, 39, 183–191. [Google Scholar] [CrossRef]
- Tian, B.; Wei, G.; Zhang, X.; Wu, Y.; Lu, G.; Tian, B.; Wei, G.; Zhang, X.; Wu, Y.; Lu, G. Water splitting over core-shell structural nanorod CdS@Cr2O3 catalyst by inhibition of H2 -O2 recombination via removing nascent formed oxygen using perfluorodecalin. Appl. Catal. B 2018, 221, 618–625. [Google Scholar] [CrossRef]
- Han, S.; Pu, Y.; Zheng, L.; Zhang, J.Z.; Fang, X. Shell-thickness dependent electron transfer and relaxation in type-II core–shell CdS/TiO2 structures with optimized photoelectrochemical performance. J. Mater. Chem. A 2015, 3, 22627–22635. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thampi, K.R.; Tayade, R.J. Visible light driven redox-mediator-free dual semiconductor photocatalytic systems for pollutant degradation and the ambiguity in applying Z-scheme concept. Appl. Catal. B 2018, 227, 296–311. [Google Scholar] [CrossRef]
- Lu, X.; Che, W.; Hu, X.; Wang, Y.; Zhang, A.; Deng, F.; Dionysiou, D.D. The facile fabrication of novel visible-light-driven Z-scheme CuInS2/Bi2WO6 heterojunction with intimate interface contact by in situ hydrothermal growth strategy for extraordinary photocatalytic performance. Chem. Eng. J. 2019, 356, 819–829. [Google Scholar] [CrossRef]
- Liang, M.; Borjigin, T.; Zhang, Y.; Liu, H.; Liu, B.; Guo, H. Z-Scheme Au@ Void@ g-C3N4/SnS Yolk–Shell Heterostructures for Superior Photocatalytic CO2 Reduction under Visible Light. ACS Appl. Mater. Interfaces 2018, 10, 34123–34131. [Google Scholar] [CrossRef]
- Ning, X.; Li, J.; Yang, B.; Zhen, W.; Li, Z.; Tian, B.; Lu, G. Inhibition of photocorrosion of CdS via assembling with thin film TiO2 and removing formed oxygen by artificial gill for visible light overall water splitting. Appl. Catal. B 2017, 212, 129–139. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Gao, X.; Yao, W.; Wei, W.; Chen, X.; Zong, R.; Zhu, Y. Core-shell g-C3N4 @ZnO composites as photoanodes with double synergistic effects for enhanced visible-light photoelectrocatalytic activities. Appl. Catal. B 2017, 217, 169–180. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, S.; Song, X.; Luo, S. Photocatalyst based on TiO2 nanotube arrays co-decorated with CdS quantum dots and reduced graphene oxide irradiated by γ rays for effective degradation of ethylene. Appl. Surf. Sci. 2018, 442, 245–255. [Google Scholar] [CrossRef]
- Hu, Z.; Quan, H.; Chen, Z.; Shao, Y.; Li, D. New insight into an efficient visible light-driven photocatalytic organic transformation over CdS/TiO2 photocatalysts. Photochem. Photobiol. Sci. 2018, 17, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Han, X.; Yu, Z.; Du, Y.; Song, B.; Xu, P. Highly efficient visible-light-driven photocatalytic hydrogen production on CdS/Cu7S4/g-C3N4 ternary heterostructures. ACS Appl. Mater. Interfaces 2018, 10, 20404–20411. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Mahadik, M.A.; Chung, H.S.; Ryu, J.H.; Jang, J.S. Facile hydrothermally synthesized a novel CdSnanoflower/Rutile-TiO2nanorodheterojunctionphotoanode used for photoelectrocatalytic hydrogen Generation. ACS Sustain. Chem. Eng. 2017, 5, 7537–7548. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Xu, P.; Ma, Z.; Wang, J.; Yuan, H. Rational design of wide spectral responsive heterostructures of au nanorods coupled Ag3PO4 with enhanced photocatalytic performance. ACS Appl. Mater. Interfaces 2017, 9, 20620–20629. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, W.; Luo, W.; Chen, X.; Zhu, Y. Ultrathin nanosheets g-C3N4@Bi2WO6 core-shell structure via low temperature reassembled strategy to promote photocatalytic activity. Appl. Catal. B 2018, 237, 633–640. [Google Scholar] [CrossRef]
- Gupta, R.; Eswar, K.R.; Modak, J.M.; Madras, G. Effect of morphology of zinc oxide in ZnO-CdS-Ag ternary nanocomposite towards photocatalytic inactivation of E. coli under UV and visible light. Chem. Eng. J. 2017, 307, 966–980. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, G.; Wu, X.; Yin, S. Novel visible-light-driven Z-scheme Bi12GeO20/g-C3N4 photocatalyst: Oxygen-induced pathway of organic pollutants degradation and proton assisted electron transfer mechanism of Cr (VI) reduction. Appl. Catal. B 2017, 207, 17–26. [Google Scholar] [CrossRef]
- Li, B.; Lai, C.; Zeng, G.; Qin, L.; Yi, H.; Huang, D.; Zhang, C. Facile Hydrothermal Synthesis of Z-scheme Bi2Fe4O9/Bi2WO6 Heterojunction Photocatalyst with Enhanced Visible-Light Photocatalytic Activity. ACS Appl. Mater. Interfaces 2018, 10, 18824–18836. [Google Scholar] [CrossRef] [PubMed]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A. Synthesis and characterizations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Cleaner Prod. 2018, 179, 42–54. [Google Scholar] [CrossRef]
- Fan, G.; Zheng, X.; Luo, J.; Peng, H.; Lin, H.; Bao, M.; Zhou, J. Rapid synthesis of Ag/AgCl@ ZIF-8 as a highly efficient photocatalyst for degradation of acetaminophen under visible light. Chem. Eng. J. 2018, 351, 782–790. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Feng, M.; Tu, W.; Xiao, T.; Xiong, T.; Chew, J.W. Visible-light-driven removal of tetracycline antibiotics and reclamation of hydrogen energy from natural water matrices and wastewater by polymeric carbon nitride foam. Water Res. 2018, 144, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, P.; Das, M.R. Hydrothermal assisted decoration of NiS2 and CoS nanoparticles on the reduced graphene oxide nanosheets for sunlight driven photocatalytic degradation of azo dye: Effect of background electrolyte and surface charge. J. Colloid Interface Sci. 2018, 516, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yu, J.; Guo, D.; Cui, C.; Ho, W. A hierarchical Z-Scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity. Small 2015, 11, 5262–5271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zong, X.; An, L.; Hua, S.; Miao, X.; Luan, S.; Wen, Y.; Tao, F.F.; Sun, Z. Consciously constructing heterojunction or direct Z-Scheme photocatalysts by regulating electron flow direction. ACS Catal. 2018, 8, 2209–2217. [Google Scholar] [CrossRef]
- Li, Q.; Guan, Z.; Wu, D.; Zhao, X.; Bao, S.; Tian, B. Z-scheme BiOCl-Au-CdSheterostructure with enhanced sunlight-driven photocatalytic activity in degrading water dyes and antibiotics. ACS Sustain. Chem. Eng. 2017, 5, 6958–6968. [Google Scholar] [CrossRef]
- Sun, C.; Xu, Q.; Xie, Y.; Ling, Y.; Hou, Y. Designed synthesis of anatase-TiO2 (B) biphase nanowires/ZnO nanoparticles heterojunction for enhanced photocatalysis. J. Mater. Chem. A 2018, 6, 8289–8298. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Yao, F.; Wang, S.; Sun, J.; An, H.; Li, X. Visible-light photocatalytic degradation of multiple antibiotics by AgI nanoparticle-sensitized Bi5O7I microspheres: Enhanced interfacial charge transfer based on Z-scheme heterojunctions. J. Catal. 2017, 352, 160–170. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Wang, H. Construction of All-Solid-State Z–scheme Photocatalyst Based on Graphite Carbon Nitride and Its Enhancement to Catalytic Activity. Environ. Sci. Nano 2018, 7, 4193–4205. [Google Scholar] [CrossRef]
- Yang, J.; Xie, T.; Liu, C.; Xu, L. Dy(III) doped BiOCl powder with superior highly visible-light-driven photocatalytic activity for rhodamine B photodegradation. Nanomaterials 2018, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, K.S.; Castillo, R.B.; Sillanpaa, M.; Kumar, R.T.R. Effective shell wall thickness of vertically aligned ZnO-ZnS core-shell nanorod arrays on visible photocatalytic and photo sensing properties. Appl. Catal. B 2018, 237, 128–139. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Sun, Z.; Pan, L. Novel Bi2MoO6/TiO2 heterostructure microspheres for degradation of benzene series compound under visible light irradiation. J. Colloid Interface Sci. 2016, 463, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Y.; Fu, W.F. Visible-light driven oxidative coupling of amines to imines with high selectivity in air over core-shell structured CdS@ C3N4. Appl. Catal. B 2018, 236, 176–183. [Google Scholar] [CrossRef]











| pH | 3 | 5 | 7 | 9 | 11 |
|---|---|---|---|---|---|
| Potential (mV) | −12.83 | −9.85 | −8.11 | −5.46 | −2.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Wu, Z.; He, X.; Yang, X.; Chen, X.; Gao, Z. Constructing a Z-scheme Heterojunction of Egg-Like Core@shell CdS@TiO2 Photocatalyst via a Facile Reflux Method for Enhanced Photocatalytic Performance. Nanomaterials 2019, 9, 222. https://doi.org/10.3390/nano9020222
Xue Y, Wu Z, He X, Yang X, Chen X, Gao Z. Constructing a Z-scheme Heterojunction of Egg-Like Core@shell CdS@TiO2 Photocatalyst via a Facile Reflux Method for Enhanced Photocatalytic Performance. Nanomaterials. 2019; 9(2):222. https://doi.org/10.3390/nano9020222
Chicago/Turabian StyleXue, Yongtao, Zhansheng Wu, Xiufang He, Xia Yang, Xiaoqing Chen, and Zhenzhen Gao. 2019. "Constructing a Z-scheme Heterojunction of Egg-Like Core@shell CdS@TiO2 Photocatalyst via a Facile Reflux Method for Enhanced Photocatalytic Performance" Nanomaterials 9, no. 2: 222. https://doi.org/10.3390/nano9020222