Photocatalytic Properties of Graphene/Gold and Graphene Oxide/Gold Nanocomposites Synthesized by Pulsed Laser Induced Photolysis
Abstract
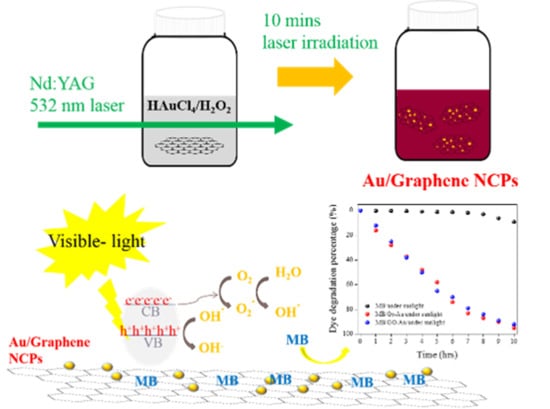
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Gr/Au and GO/Au NCP Syntheses
2.4. Photocatalytic Activity Test
3. Results and Discussion
3.1. Laser-Induced Photolysis and Formation of Au NPs
3.2. Stability of Synthesized NCP
3.3. Characterization of Synthesized NCPs
3.4. Photocatalytic Activity of Synthesized Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Anjaneyulu, R.B.; Mohan, B.S.; Naidu, G.P.; Muralikrishna, R. Visible light enhanced photocatalytic degradation of methylene blue by ternary nanocomposite, MoO3/Fe2O3/rGO. J. Asian Ceram. Soc. 2018, 6, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xu, L.; Wang, H.; Wang, W.; Zhang, L. TiO2/graphene porous composite and its photocatalytic degradation of methylene blue. Mater. Des. 2016, 108, 632–639. [Google Scholar] [CrossRef]
- Benjwal, P.; Kumar, M.; Chamoli, P.; Kar, K.K. Enhanced photocatalytic degradation of methylene blue and adsorption of arsenic (iii) by reduced graphene oxide (rGO)–metal oxide (TiO2/Fe3O4) based nanocomposites. RSC Adv. 2015, 5, 73249–73260. [Google Scholar] [CrossRef]
- Roushani, M.; Mavaei, M.; Daneshfar, A.; Rajabi, H.R. Application of graphene quantum dots as green homogenous nanophotocatalyst in the visible-light-driven photolytic process. J. Mater. Sci. Mater. Electron. 2017, 28, 5135–5143. [Google Scholar] [CrossRef]
- Nuengmatcha, P.; Porrawatkul, P.; Chanthai, S.; Sricharoen, P.; Limchoowong, N. Enhanced photocatalytic degradation of methylene blue using Fe2O3/graphene/CuO nanocomposites under visible light. J. Environ. Chem. Eng. 2019, 7, 103438. [Google Scholar] [CrossRef]
- LimaBeluci, N.; Mateus, G.A.P.; Miyashiro, C.S.; Homem, N.C.; Gomes, R.G.; Fagundes-Klen, M.R.; Bergamasco, R.; Vieira, A.M.S. Hybrid treatment of coagulation/flocculation process followed by ultrafiltration in TiO2-modified membranes to improve the removal of reactive black 5 dye. Sci. Total Environ. 2019, 664, 222–229. [Google Scholar]
- Fayoud, N.; Tahiri, S.; Alami Younssi, S.; Albizane, A.; Gallart- Mateu, D.; Cervera, M.L.; de la Guardia, M. Kinetic, isotherm and thermodynamic studies of the adsorption of methylene blue dye onto agro-based cellulosic materials. Desalin. Water Treat. 2016, 57, 16611–16625. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Dong, Y.; Li, H.; Xia, Y.; Wang, H. One-step synthesis of Bi2MoO6/reduced graphene oxide aerogel composite with enhanced adsorption and photocatalytic degradation performance for methylene blue. Mater. Sci. Semicon. Proc. 2018, 88, 214–223. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Z.; Xu, L.; Wang, H.; Fu, N. Preparation of reduced graphene oxide/meso-TiO2/AuNPs ternary composites and their visible-light-induced photocatalytic degradation n of methylene blue. Appl. Surf. Sci. 2016, 369, 576–583. [Google Scholar] [CrossRef]
- Echabbi, F.; Hamlich, M.; Harkati, S.; Jouali, A.; Tahiri, S.; Lazar, S.; Lakhmiri, R.; Safi, M. Photocatalytic degradation of methylene blue by the use of titanium-doped Calcined Mussel Shells CMS/TiO2. J. Environ. Chem. Eng. 2019, 7, 103293. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, S.; Fu, Z. Preparation of Multicycle GO/TiO2 Composite Photocatalyst and Study on Degradation of Methylene Blue Synthetic Wastewater. Appl. Sci. 2019, 9, 3282. [Google Scholar] [CrossRef] [Green Version]
- Atout, H.; Álvarez, M.G.; Chebli, D.; Bouguettoucha, A.; Tichit, D.; Llorca, J.; Medina, F. Enhanced photocatalytic degradation of methylene blue: Preparation of TiO2/reduced graphene oxide nanocomposites by direct sol-gel and hydrothermal methods. Mater. Sci. Bull. 2019, 95, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Cho, B.; Ko, W. Photocatalytic degradation of methylene blue by graphene impregnated with CeO2 nanoparticles under ultrasonic irradiation. Asian J. Chem. 2013, 25, 8178–8180. [Google Scholar] [CrossRef]
- Seema, H.; Kemp, K.C.; Chandra, V.; Kim, K.S. Graphene–SnO2 composites for highly efficient photocatalytic degradation of methylene blue under sunlight. Nanotechnology 2012, 23, 355705. [Google Scholar] [CrossRef]
- Khoa, N.T.; Kim, S.W.; Yoo, D.; Cho, S.; Kim, E.J.; Hahn, S.H. Fabrication of Au/graphene-wrapped ZnO-nanoparticle-assembled hollow spheres with effective photoinduced charge transfer for photocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 3524–3531. [Google Scholar] [CrossRef]
- Khalil, I.; Rahmati, S.; Julkapli, N.M.; Yehye, W.A. Graphene metal nanocomposites—Recent progress in electrochemical biosensing applications. J. Indust. Eng. Chem. 2018, 59, 425–439. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene–gold nanoparticles hybrid—synthesis, functionalization, and application in a electrochemical and surface-enhanced raman scattering biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Zhao, X.; Yang, J.; Shan, X.; Yang, L.; Zhang, Y.; Li, X.; Gao, M. ZnO–graphene composite for photocatalytic degradation of methylene blue dye. Cataly. Comm. 2012, 29, 29–34. [Google Scholar] [CrossRef]
- Atchudan, R.; Immanuel Edison, T.N.J.; Perumal, S.; Karthikeyan, D.; Lee, Y.R. Facile synthesis of zinc oxide nanoparticles decorated graphene oxide composite via simple solvothermal route and their photocatalytic activity on methylene blue degradation. J. Photochem. Photobiol. B Biol. 2016, 162, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhang, L.L.; Ma, J.; Zhao, X.S. Photocatalytic degradation of dyes over graphene–gold nanocomposites under visible light irradiation. Chem. Commun. 2010, 46, 6099–6101. [Google Scholar] [CrossRef] [PubMed]
- Šimšíková, M.; Bartoš, M.; Keša, P.; Šikola, T. Green approach for preparation of reduced graphene oxide decorated with gold nanoparticles and its optical and catalytic properties. Mater. Chem. Phys. 2016, 177, 339–345. [Google Scholar] [CrossRef]
- Hurtado, R.B.; Cortez-Valadez, M.; Aragon-Guajardo, J.R.; Cruz-Rivera, J.J.; Martínez-Suárez, F.; Flores-Acosta, M. One-step synthesis of reduced graphene oxide/gold nanoparticles under ambient conditions. Arabian J. Chem. 2020, 13, 1633–1640. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Grácio, J. Surface modification of graphene nanosheets with gold nanoparticles: The role of oxygen moieties at graphene surface on gold nucleation and growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Chuang, M.-K.; Lin, S.-W.; Chen, F.-C.; Chu, C.-W.; Hsu, C.-S. Gold nanoparticle-decorated graphene oxides for plasmonic-enhanced polymer photovoltaic devices. Nanoscale 2014, 6, 1573–1579. [Google Scholar] [CrossRef]
- Moussa, S.; Atkinson, G.; El-Shall, M.S.; Shehata, A.; AbouZeid, K.M.; Mohamed, M.B. Laser assisted photocatalytic reduction of metal ions by graphene oxide. J. Mater. Chem. 2011, 21, 9608–9619. [Google Scholar] [CrossRef]
- Zhao, C.; Qu, S.; Qiu, J.; Zhu, C. Photoinduced formation of colloidal Au by a near-infrared femtosecond laser. J. Mater. Res. 2003, 18, 1710–1714. [Google Scholar] [CrossRef]
- Kurihara, K.; Kizling, J.; Stenius, P.; Fendler, J.H. Laser and pulse radiolytically induced colloidal gold formation in water and in water-in-oil microemulsions. J. Am. Chem. Soc. 1983, 105, 2574–2579. [Google Scholar] [CrossRef]
- Bronstein, L.; Chernyshov, D.; Valetsky, P.; Tkachenko, N.; Lemmetyinen, H.; Hartmann, J.; Förster, S. Laser photolysis formation of gold colloids in block copolymer micelles. Langmuir 1999, 15, 83–91. [Google Scholar] [CrossRef]
- Kuladeep, R.; Jyothi, L.; Shadak Alee, K.; Deepak, K.L.N.; Narayana Rao, D. Laser-assisted synthesis of Au-Ag alloy nanoparticles with tunable surface plasmon resonance frequency. Opt. Mater. Express 2012, 2, 161–172. [Google Scholar] [CrossRef]
- Shirk, M.D.; Molian, P.A. A review of ultrashort pulsed laser ablation of materials. J. Laser Appl. 1998, 10, 18–28. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.-W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via laser ablation/irradiation in liquid: A review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Kubiliūtė, R.; Maximova, K.A.; Lajevardipour, A.; Yong, J.; Hartley, J.S.; Mohsin, A.S.M.; Blandin, P.; Chon, J.; Sentis, M.; Stoddart, P.R.; et al. Ultra-pure, water-dispersed Au nanoparticles produced by femtosecond laser ablation and fragmentation. Int. J. Nanomed. 2013, 8, 2601–2611. [Google Scholar]
- Yu, Y.; Yan, L.; Si, J.; Xu, Y.; Hou, X. Femtosecond laser assisted synthesis of gold nanorod and graphene hybrids and its photothermal property in the near-infrared region. J. Phys. Chem. Solids 2019, 132, 116–120. [Google Scholar] [CrossRef]
- McGilvray, K.L.; Granger, J.; Correia, M.; Banks, J.T.; Scaiano, J.C. Opportunistic use of tetrachloroaurate photolysis in the generation of reductive species for the production of gold nanostructures. Phys. Chem. Chem. Phys. 2011, 13, 11914–11918. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Chou, C.-M.; Tsai, K.-L.; Hsu, S.; Yehye, W.A.; Hsiao, V.K.S. Gold Nanofilm-Coated Porous Silicon as Surface-Enhanced Raman Scattering Substrate. Appl. Sci. 2019, 9, 4806. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, F.; Razi, S.; Madanipour, K. Single-step laser-assisted graphene oxide reduction and nonlinear optical properties exploration via CW laser excitation. J. Electron. Mater. 2018, 47, 2871–2879. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wei, B. Hybrid nanostructures of metal/two-dimensional nanomaterials for plasmon-enhanced applications. Chem. Soc. Rev. 2016, 45, 3145–3187. [Google Scholar] [CrossRef] [Green Version]
- Ren, R.; Li, S.; Li, J.; Ma, J.; Liu, H.; Ma, J. Enhanced catalytic activity of Au nanoparticles self-assembled on thiophenol functionalized graphene. Catal. Sci. Technol. 2015, 5, 2149–2156. [Google Scholar] [CrossRef]
- Biroju, R.K.; Choudhury, B.; Giri, P.K. Plasmon-enhanced strong visible light photocatalysis by defect engineered CVD graphene and graphene oxide physically functionalized with Au nanoparticles. Catal. Sci. Technol. 2016, 6, 7101–7112. [Google Scholar] [CrossRef]
- Movahed, S.K.; Fakharian, M.; Dabiri, M.; Bazgir, A. Gold nanoparticle decorated reduced graphene oxide sheets with high catalytic activity for ullmann homocoupling. RSC Adv. 2014, 4, 5243–5247. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-H.; Shen, H.-T.; Chang, W.-H.; Khalil, I.; Liao, S.-Y.; A. Yehye, W.; Liu, S.-C.; Chu, C.-C.; Hsiao, V.K.S. Photocatalytic Properties of Graphene/Gold and Graphene Oxide/Gold Nanocomposites Synthesized by Pulsed Laser Induced Photolysis. Nanomaterials 2020, 10, 1985. https://doi.org/10.3390/nano10101985
Chen L-H, Shen H-T, Chang W-H, Khalil I, Liao S-Y, A. Yehye W, Liu S-C, Chu C-C, Hsiao VKS. Photocatalytic Properties of Graphene/Gold and Graphene Oxide/Gold Nanocomposites Synthesized by Pulsed Laser Induced Photolysis. Nanomaterials. 2020; 10(10):1985. https://doi.org/10.3390/nano10101985
Chicago/Turabian StyleChen, Li-Hsiou, Huan-Ting Shen, Wen-Hsin Chang, Ibrahim Khalil, Su-Yu Liao, Wageeh A. Yehye, Shih-Chuan Liu, Chih-Chien Chu, and Vincent K. S. Hsiao. 2020. "Photocatalytic Properties of Graphene/Gold and Graphene Oxide/Gold Nanocomposites Synthesized by Pulsed Laser Induced Photolysis" Nanomaterials 10, no. 10: 1985. https://doi.org/10.3390/nano10101985