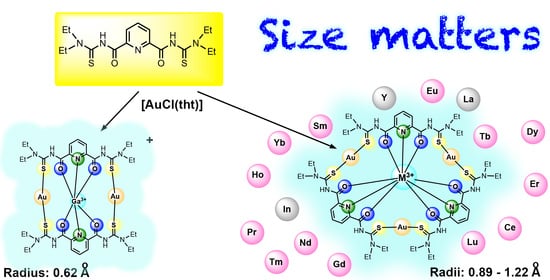
Gold-Based Coronands as Hosts for M3+ Metal Ions: Ring Size Matters
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Synthetic Aspects
2.2. Lanthanum and the Lanthanide Series
2.3. Group 3 and Group 13 Elements
2.4. More Structural Aspects and Potential Implication for the Reactivity
3. Materials and Methods
3.1. Syntheses
3.2. Spectroscopic Methods
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives; Wiley VCH: Weinheim, Germany, 1995. [Google Scholar]
- Lehn, J.-M. Toward complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. USA 2002, 99, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Philip, D.; Stoddart, J.F. Self-Assembly in Natural and Unnatural Systems. Angew. Chem. Int. Ed. 1996, 35, 1154–1196. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Wilmer, C.E.; Ki, J.; Browne, K.P.; Bishop, K.J.M. Self-assembly: From crystals to cells. Soft Matter 2009, 5, 1110–1128. [Google Scholar] [CrossRef]
- Pochan, D.; Scherman, O. Introduction: Molecular Self-Assembly. Chem. Rev. 2021, 121, 13699–13700. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal–Organic Frameworks and Self-Assembled Supramolecular Coordination Complexes: Comparing and Contrasting the Design, Synthesis, and Functionality of Metal–Organic Materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [Green Version]
- Rissanen, K.; Barbour, L.J.; McGillivray, L.R. Structural macrocyclic supramolecular chemistry. CrystEngComm 2014, 16, 3644–3645. [Google Scholar] [CrossRef]
- Schulze, B.; Schubert, U.S. Beyond click chemistry—Supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef]
- Muller, T.; Bräse, S. Tetrahedral organic molecules as components in supramolecular architectures and in covalent assemblies, networks and polymers. RSC Adv. 2014, 4, 6886–6907. [Google Scholar] [CrossRef]
- Constable, E.C. Stereogenic metal centres—From Werner to supramolecular chemistry. Chem. Soc. Rev. 2013, 42, 1637–1651. [Google Scholar] [CrossRef]
- Saalfrank, R.W.; Scheurer, A. Coronates, Spherical Containers, Bowl-Shaped Surfaces, Porous 1D-, 2D-, 3D-Metallo-Coordination Polymers, and Metallodendrimers. Top. Curr. Chem. 2012, 319, 125–170. [Google Scholar]
- Lehn, J.-M. Par-delà la synthèse: L’auto-organisation. Beyond synthesis: Self-organization. Comptes Rendus Chim. 2011, 14, 348–361. [Google Scholar] [CrossRef]
- Aromi, G.; Gamez, P.; Reedijk, J. Poly beta-diketones: Prime ligands to generate supramolecular metalloclusters. Coord. Chem. Rev. 2008, 252, 964–989. [Google Scholar] [CrossRef]
- Saalfrank, R.W.; Maid, H.; Scheurer, A. Supramolecular Coordination Chemistry: The Synergistic Effect of Serendipity and Rational Design. Angew. Chem. Int. Ed. 2008, 47, 8794–8824. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, G.F.; Malefetse, T.J. New Self-Assembled Structural Motifs in Coordination Chemistry. Chem. Rev. 2000, 100, 3483–3537. [Google Scholar] [CrossRef]
- Moreno-Alcantar, G.; Casini, A. Bioinorganic supramolecular coordination complexes and their biomedical applications. FEBS Lett. 2023, 597, 191–202. [Google Scholar] [CrossRef]
- Leininger, S.; Olenyuk, B.; Stang, P.J. Self-Assembly of Discrete Cyclic Nanostructures Mediated by Transition Metals. Chem. Rev. 2000, 100, 853–908. [Google Scholar] [CrossRef]
- Fujita, M.; Umemoto, K.; Yishizawa, M.; Fujita, M.; Kusukawa, T.; Biradha, K. Molecular Paneling via coordination. Chem. Commun. 2001, 509–518. [Google Scholar] [CrossRef]
- Caulder, D.L.; Raymond, K. The rational design of high symmetry coordination clusters. J. Chem. Soc. Dalton Trans. 1999, 1185–1200. [Google Scholar] [CrossRef]
- Ganneschi, N.C.; Masar, M.S.; Mirkin, C.A. Development of a Coordination Chemistry-Based Approach for Functional Supramolecular Structures. Acc. Chem. Res. 2005, 38, 825–837. [Google Scholar] [CrossRef]
- Cotton, F.A.; Lin, C.; Murillo, C.A. Supramolecular Arrays Based on Dimetal Building Units. Acc. Chem. Res. 2001, 34, 759–771. [Google Scholar] [CrossRef]
- Kryshenko, Y.K.; Seidel, S.R.; Arif, A.M.; Stang, P.J. Coordination-Driven Self-Assembly of Predesigned Supramolecular Triangles. J. Am. Chem. Soc. 2003, 125, 5193–5198. [Google Scholar] [CrossRef] [PubMed]
- Cotton, F.A.; Lin, C.; Murillo, C.A. Supramolecular Squares with Mo24+ Corners. Inorg. Chem. 2001, 40, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Caulder, D.L.; Powers, R.E.; Parac, T.N.; Raymond, K.N. The Self-Assembly of a Predesigned Tetrahedral M4L6 Supramolecular Cluster. Angew. Chem. Int. Ed. 1998, 37, 1840–1843. [Google Scholar] [CrossRef]
- Fujita, M.; Oguro, D.; Miyazawa, M.; Oka, H.; Yamaguchi, K.; Ogura, K. Self-assembly of ten molecules into nanometer-sized organic host frameworks. Nature 1995, 378, 469–471. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Beyer, L.; Hoyer, E.; Liebscher, J.; Hartmann, H. Komplexbildung mit N-Acyl-Thioharnstoffen. Z. Chem. 1981, 21, 81–91. [Google Scholar] [CrossRef]
- Koch, K.R. New chemistry with old ligands: N-alkyl- and N,N-dialkyl-N′-acyl(aroyl)thioureas in co-ordination, analytical and process chemistry of the platinum group metals. Coord. Chem. Rev. 2001, 216–217, 473–488. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Abram, U. Rhenium and Technetium Complexes with N,N-Dialkyl-N’-benzoylthioureas. Inorg. Chem. 2007, 46, 5319. [Google Scholar]
- Sucena, S.F.; Pham, T.T.; Hagenbach, A.; Pham, C.T.; Abram, U. Structural Diversity of Alkaline Earth Centered Gold(I) Metallacoronates. Eur. J. Inorg. Chem. 2020, 2020, 4341–4349. [Google Scholar] [CrossRef]
- Selvakumaran, N.; Bhuvanesh, N.S.P.; Karvembu, R. Self-assembled Cu(II) and Ni(II) metallamacrocycles formed from 3,3,3′,3′-tetrabenzyl-1,1′-aroylbis(thiourea) ligands: DNA and protein binding studies, and cytotoxicity of trinuclear complexes. Dalton Trans. 2014, 43, 16395–16410. [Google Scholar] [CrossRef] [PubMed]
- Richter, R.; Sieler, J.; Köhler, R.; Hoyer, E.; Beyer, L.; Hansen, L.K. Kristall- und Molekülstruktur eines neuartigen Trimetallamacrocyclus: Cyclo-Tri[nickel-μ-[1,1,1′,1′-tetraethyl-3,3′-terephthaloyl-bis-thioureato(2-)-S,O:O′,S′]]. Z. Anorg. Allg. Chem. 1989, 578, 191–197. [Google Scholar] [CrossRef]
- Koch, K.R.; Bourne, S.A.; Coetzee, A.; Miller, J. Self-assembly of 2:2 and 3:3 metallamacrocyclic complexes of platinum(II) with symmetrical, bipodal N′,N′,N‴N‴-tetraalkyl-N,N″-phenylenedicarbonylbis(thiourea). J. Chem. Soc. Dalton Trans. 1999, 3157–3161. [Google Scholar] [CrossRef]
- Rodenstein, A.; Griebel, J.; Richter, R.; Kirmse, R. Synthese, Struktur und EPR-Untersuchungen von binuklearen Bis(N,N,N‴,N‴-tetraisobutyl-N′,N″-isophthaloylbis(thioureato))-Komplexen des CuII, NiII, ZnII, CdII und PdII. Z. Anorg. Allg. Chem. 2008, 634, 867–874. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Pham, C.T.; Rodenstein, A.; Kirmse, R.; Abram, U. Bipodal Acylthiourea Ligands as Building Blocks for Bi-, Tetra-, and Polynuclear Oxorhenium(V) Complexes. Inorg. Chem. 2011, 50, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.R.; Hallale, O.; Bourne, S.A.; Miller, J.; Bacsa, J. Self-assembly of 2:2 metallomacrocyclic complexes of NiII and PdII with 3,3,3′,3′-tetraalkyl-1,1′-isophthaloylbis(thioureas). Crystal and molecular structures of cis-[Pd(L2-S,O)]2 and the adducts of the corresponding NiII complexes: [Ni(L1-S,O)(pyridine)2]2 and [Ni(L1-S,O)(4-dimethylaminopyridine)2]2. J. Mol. Struct. 2001, 561, 185–196. [Google Scholar]
- Bourne, S.A.; Hallale, O.; Koch, K.R. Hydrogen-Bonding Networks in a Bipodal Acyl-thiourea and Its NiII 2:2 Metallamacrocyclic Complex. Cryst. Growth Des. 2005, 5, 307–312. [Google Scholar] [CrossRef]
- Rodenstein, A.; Richter, R.; Kirmse, R. Synthese und Struktur von N,N,N‴,N‴-Tetraisobutyl-N′,N″-isophthaloylbis(thioharnstoff) und Dimethanol-bis(N,N,N‴,N‴-tetraisobutyl-N′,N″-isophthaloylbis(thioureato))dicobalt(II). Z. Anorg. Allg. Chem. 2007, 633, 1713–1717. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Jegathesh, J.J.; Takiden, A.; Hauenstein, D.; Pham, C.T.; Le, C.D.; Abram, U. 2,6-Dipicolinoylbis(N,N-dialkylthioureas) as versatile building blocks for oligo- and polynuclear architectures. Dalton Trans. 2016, 45, 10771–10779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, C.T.; Nguyen, H.H.; Hagenbach, A.; Abram, U. Iron(III) Metallacryptand and Metallacryptate Assemblies Derived from Aroylbis(N,N-diethylthioureas). Inorg. Chem. 2017, 56, 11406–11416. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.T.; Nguyen, H.H.; Abram, U. AgI Metallacoronates and HgII Metallacryptates Derived from a Catechol-Based Aroylbis(N,N-diethylthiourea). Eur. J. Inorg. Chem. 2018, 2018, 951–957. [Google Scholar] [CrossRef]
- Pham, C.T.; Roca Jungfer, M.; Abram, U. Indium(III) {2}-metallacryptates assembled from 2,6-dipicolinoyl-bis(N,N-diethylthiourea). New J. Chem. 2020, 44, 3672–3680. [Google Scholar] [CrossRef]
- Jesudas, J.J.; Pham, C.T.; Hagenbach, A.; Abram, U.; Nguyen, H.H. Trinuclear CoIILnIIICoII Complexes (Ln = La, Ce, Nd, Sm, Gd, Dy, Er, and Yb) with 2,6-Dipicolinoylbis(N,N-diethylthiourea): Synthesis, Structures, and Magnetism. Inorg. Chem. 2020, 59, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Le, C.D.; Pham, C.T.; Nguyen, H.H. Zinc(II) {2}-metallacoronates and {2}-metallacryptates based on dipicolinoylbis(N,N-diethylthiourea): Structures and biological activities. Polyhedron 2019, 173, 114143. [Google Scholar] [CrossRef]
- Pham, C.T.; Nguyen, T.H.; Matsumoto, K.; Nguyen, H.H. CuI/CuII Complexes with Dipicolinoylbis(N,N-diethylthiourea): Structures, Magnetism, and Guest Ion Exchange. Eur. J. Inorg. Chem. 2019, 2019, 4142–4146. [Google Scholar] [CrossRef]
- Pham, C.T.; Nguyen, T.H.; Trieu, T.N.; Matsumoto, K.; Nguyen, H.H. Syntheses, Structures, and Magnetism of Trinuclear Zn2Ln Complexes with 2,6-Dipicolinoylbis(N,N-diethylthiourea). Z. Anorg. Allg. Chem. 2019, 645, 1072–1078. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Abram, U.; Pham, C.T. Ammonium-Iron(III) metallacryptate inclusion complexes based on Aroylbis(N,N-diethylthioureas): Synthesis and structure. Vietnam. J. Chem. 2022, 60, 622–628. [Google Scholar]
- Luckay, R.C.; Mebrahtu, F.; Esterhuysen, C.; Koch, K.R. Extraction and transport of gold(III) using some acyl(aroyl)thiourea and a crystal structure of one of the complexes. Inorg. Chem. Commun. 2010, 13, 468–470. [Google Scholar] [CrossRef]
- Bensch, W.; Schuster, M. Komplexierung von Gold mit N,N-Dialkyl-N’-benzoylthioharnstoffen: Die Kristallstruktur von N,N-Diethyl-N’benzoylthioureatogold(I)-chlorid. Z. Anorg. Allg. Chem. 1992, 611, 99–102. [Google Scholar] [CrossRef]
- Pathaneni, S.S.; Desiraju, G.R. Database analysis of Au…Au interactions. J. Chem. Soc. Dalton Trans. 1993, 319–322. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Schwade, V.D.; Kirsten, L.; Hagenbach, A.; Lang, E.S.; Abram, U. Indium(III), lead(II), gold(I) and copper(II) complexes with isophthaloylbis(thiourea) ligands. Polyhedron 2013, 55, 155–161. [Google Scholar] [CrossRef]
- Gutíerrez, A.; Bernal, J.; Villacampa, M.D.; Cativiela, C.; Laguna, A.; Gimeno, M.C. Synthesis of new gold(I) thiolates containing amino acid moieties with potential biological interest. Inorg. Chem. 2013, 52, 6473–6480. [Google Scholar] [CrossRef]
- Kleinpeter, E.; Beyer, L. 1H-NMR-Untersuchung der behinderten Rotation um die C-N-Bindung in 1,1’-Diäthyl-3-benzoylharnstoff-Derivaten. J. Prakt. Chem. 1975, 317, 938. [Google Scholar] [CrossRef]
- Shanon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layfield, R.A. Lanthanides. In Comprehensive Coordination Chemistry III; Constable, E., Parkin, G., Que, L., Jr., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Bernot, K.; Daiguebonne, C.; Calvez, G.; Suffren, Y.; Guillou, O. A Journey in Lanthanide Coordination Chemistry: From Evaporable Dimers to Magnetic Materials and Luminescent Devices. Acc. Chem. Res. 2021, 54, 427–440. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Review: Lanthanide coordination chemistry: From old concepts to coordination polymers. J. Coord. Chem. 2014, 67, 3704–3733. [Google Scholar] [CrossRef]
- Bell, D.J.; Natrajan, L.S.; Riddell, I.A. Design of lanthanide based metal–organic polyhedral cages for application in catalysis, sensing, separation and magnetism. Coord. Chem. Rev. 2022, 472, 214786. [Google Scholar] [CrossRef]
- Roesky, P.W. Molecular Catalysis of Rare-Earth Elements. Struct. Bond. 2010, 137, 1–228. [Google Scholar]
- Shibasaki, M.; Yoshikawa, N. Lanthanide Complexes in Multifunctional Asymmetric Catalysis. Chem. Rev. 2002, 102, 2178–2210. [Google Scholar] [CrossRef]
- Inanaga, J.; Furuno, H.; Hayano, T. Asymmetric Catalysis and Amplification with Chiral Lanthanide Complexes. Chem. Rev. 2002, 102, 2211–2226. [Google Scholar] [CrossRef] [PubMed]
- Edelman, F.T. Lanthanide amidinates and guanidinates in catalysis and materials science: A continuing success story. Chem. Soc. Rev. 2012, 41, 7657–7672. [Google Scholar] [CrossRef] [PubMed]
- Kazeminejad, N.; Munzel, D.; Gamer, M.T.; Roesky, P.W. Bis(amidinate) ligands in early lanthanide chemistry—Synthesis, structures, and hydroamination catalysis. Chem. Commun. 2017, 53, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.B.; Seo, S.; Marks, T.J. Organo-fn,d0-Mediated Synthesis of Amine-Capped Polyethylenes. Scope and Mechanism. Organometallics 2008, 27, 2411–2420. [Google Scholar] [CrossRef]
- Amin, S.B.; Marks, T.J. Versatile Pathways for In Situ Polyolefin Functionalization with Heteroatoms: Catalytic Chain Transfer. Angew. Chem. Int. Ed. 2008, 47, 2006–2025. [Google Scholar] [CrossRef]
- Liu, H.; Eisen, M.S. Organo-f-Complexes for Efficient and Selective Hydroborations. Synthesis 2020, 52, 629–644. [Google Scholar] [CrossRef]
- Trifonov, A.A.; Basalov, I.V.; Kissel, A.A. Use of organolanthanides in the catalytic intermolecular hydrophosphination and hydroamination of multiple C–C bonds. Dalton Trans. 2016, 45, 19172–19193. [Google Scholar] [CrossRef]
- Weiss, C.J.; Marks, T.J. Organo-f-element catalysts for efficient and highly selective hydroalkoxylation and hydrothiolation. Dalton Trans. 2010, 39, 6576–6588. [Google Scholar] [CrossRef]
- Bünzli, J.-G.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of lanthanide complexes: From fundamental to prospective approaches relates to water. And molecular stimuli. J. Photochem. Photobiol C Photochem Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Kido, J.; Okamoto, Y. Organo Lanthanide Metal Complexes for Electroluminescence Materials. Chem. Rev. 2002, 102, 235–2368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Leaño, J.L., Jr.; Liu, Z.; Jin, D.; Wong, K.-L.; Liu, R.-S.; Bünzli, J.-C.G. Impact of Lanthanide Nanomaterials on Photonic Devices and Smart Applications. Small 2018, 14, 1801882. [Google Scholar] [CrossRef] [PubMed]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef]
- Marin, R.; Brunet, G.; Murugesu, M. Shining New Light on Multifunctional Lanthanide Single-Molecule Magnets. Ang. Chem. Int. Ed. 2021, 60, 1728–1746. [Google Scholar] [CrossRef]
- Fricker, S.P. The therapeutic application of lanthanides. Chem. Soc. Rev. 2006, 35, 524–533. [Google Scholar] [CrossRef]
- Cotruvo, J.A., Jr. The Chemistry of Lanthanides in Biology: Recent Discoveries, Emerging Principles, and Technological Applications. ACS Cent. Sci. 2019, 5, 1496–1506. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Luo, Z.; Qin, X.; Chen, Q.; Liu, X. Lanthanide-Activated Nanoparticles: A Toolbox for Bioimaging, Therapeutics, and Neuromodulation. Acc. Chem. Res. 2020, 17, 2692–2704. [Google Scholar] [CrossRef]
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef]
- Liu, W.; Gust, R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef]
- Da Silva Maia, P.I.; Abram, U. Gold(III) complexes in medicinal chemistry. Fut. Med. Chem. 2014, 6, 1515–1536. [Google Scholar] [CrossRef]
- Jürgens, S.; Kühn, F.E.; Casini, A. Cyclometalated Complexes of Platinum and Gold with Biological Properties: State-of-the-Art and Future Perspectives. Curr. Med. Chem. 2018, 12, 437–461. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, X.; Chang, X.; Liang, Z.; Lv, L.; Lu, Q.; We, Z.; Gust, R.; Liu, W. Recent developments of gold(I) and gold(III) complexes as therapeutic agents for cancer diseases. Chem. Soc. Rev. 2022, 51, 5518–5556. [Google Scholar] [CrossRef]
- Yue, S.; Luo, M.; Liu, H.; Wei, S. Recent Advances of Gold Compounds in Anticancer Immunity. Front. Chem. 2020, 8, 543. [Google Scholar] [CrossRef]
- Moreno-Alcantar, G.; Picchetti, P.; Casii, A. Gold Complexes in Anticancer Therapy: From New Design Principles to Particle-Based Delivery Systems. Angew. Chem. Int. Ed. 2023, 62, e202218000. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Casini, A.; Kühn, F.E. Self-assembled M2L4 coordination cages: Synthesis and potential applications. Coord. Chem. Rev. 2014, 275, 19–36. [Google Scholar] [CrossRef]
- Pöthig, A.; Casini, A. Recent Developments of Supramolecular Metal-based Structures for Applications in Cancer Therapy and Imaging. Theranostics 2019, 9, 3150–3169. [Google Scholar] [CrossRef]
- Dummert, S.V.; Saini, H.; Hussain, M.Z.; Yadava, K.; Jayaramuli, K.; Casini, A.; Fischer, R.A. Cyclodextrin metal-organic frameworks and derivatives: Recent developments and applications. Chem. Soc. Rev. 2022, 51, 5175–5213. [Google Scholar] [CrossRef]
- Baitullina, A.; Claude, G.; Sucena, S.F.; Nisli, E.; Scholz, C.; Amtauer, H.; Brenner, W.; Geppert, C.; Gorges, C.; Abram, U.; et al. Metallacages with 2,6-Dipicolinoylbis(N,N-dialkylthioureas) as novel platforms in nuclear medicine for 68Ga, 177Lu and 198Au. J. Med. Chem. 2023. submitted. [Google Scholar]
- Binnemans, K. Interpretation of europium(III) complexes. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Werts, M.H.V.; Jukes, R.T.F.; Verhoeven, J.W. The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys. Chem. Chem. Phys. 2002, 4, 1542–1548. [Google Scholar] [CrossRef]
- Ruiz-Martinez, A.; Casanova, D.; Alvarez, S. Polyhedral Structures with an Odd Number of Vertices: Nine-Coordinate Metal Compounds. Chem. Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Spectral Intensities of Trivalent Lanthanides and Actinides in Solution. II. Pm3+, Sm3+, Eu3+, Gd3+, Tb3+, Dy3+ and Ho3+. J. Chem. Phys. 1968, 49, 4412–4423. [Google Scholar] [CrossRef]
- Tanase, S.; Gallego, P.M.; de Gelder, R.; Fu, W.T. Synthesis, crystal structure and photophysical properties of europium (III) and terbium (III) complexes with pyridine-2, 6-dicarboxamide. Inorg. Chim. Acta 2007, 360, 102–108. [Google Scholar] [CrossRef]
- Zabrodsky, H.; Peleg, S.; Avnir, D. Continuous Symmetry Measures. J. Am Chem. Soc. 1992, 114, 7843–7851. [Google Scholar] [CrossRef]
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. 5. The Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef]
- Cambridge Structural Database; Version 4.23; The Cambridge Crystallographic Data Centre: Cambridge, UK, 2022.
- Volkringer, C.; Loiseau, T.; Guillou, N.; Ferey, G.; Elkaim, F.; Vimont, A. XRD and IR structural investigations of a particular breathing effect in the MOF-type gallium terephthalate MIL-53(Ga). Dalton Trans. 2009, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Fan, R.Q.; Quang, L.S.; Li, W.Q.; Wang, P.; Zhang, H.J.; Yang, Y.L. Tunable luminescence from rare 2D Ga(iii)/In(iii) coordination polymers coexisting with three different conjugated system aromatic ligands. Chem. Commun. 2014, 50, 5023–5026. [Google Scholar] [CrossRef]
- Bourque, J.L.; Nanni, R.A.; Biesinger, M.C.; Baines, K.M. Synthesis and Reactivity of Cationic Gallium(I) [12]Crown-4 Complexes. Inorg. Chem. 2021, 60, 14713–14720. [Google Scholar] [CrossRef]
- Cepeda, J.; Beobide, G.; Luque, A.; Perez-Yanez, S.; Roman, P. Structure-Directing Effect of Organic Cations in the Assembly of Anionic In(III)/Diazinedicarboxylate Architectures. Cryst. Growth Des. 2012, 12, 1501–1512. [Google Scholar] [CrossRef]
- Aghabozorg, H.; Ghadermazi, M.; Sheshmani, S.; Nakhjavan, B. Tris(piperazinediium) bis[tris(pyridine-2,6-dicarboxylato)-κ6O,N,O′;κ2O,N-indate(III)] dodecahydrate. Acta Cryst. 2006, E62, m2371–m2373. [Google Scholar] [CrossRef] [Green Version]
- Hegetschweiler, K.; Ghisletta, M.; Fässler, T.F.; Nesper, R. Hydrolysis to a Complex with a Central, Octahedral (μ6-O)In6 Unit. Angew. Chem. Int. Ed. 1993, 32, 1426–1428. [Google Scholar] [CrossRef]
- Arif, A.M.; Hart, F.A.; Thornton-Pett, M.; Zhu, W. The complex chemistry of scandium. Part 1. Preparation and properties of some scandium(III) complexes of polyamines. X-ray crystal structures of tri(nitrato-OO′)(2,2′:6′,2″-terpyridyl-NN′N″)scandium(III) and [1,2-bis(pyridine-α-carbaldimino)ethane-NN′N″N‴]-di-µ-hydroxo-di(nitrato-OO′)discandium(III) dinitrate bis(acetonitrile). J. Chem. Soc. Dalton Trans. 1984, 2449–2454. [Google Scholar]
- Kovacs, A.; Apostolidis, G.; Walther, O. Comparative Study of Complexes of Rare Earths and Actinides with 2,6-Bis(1,2,4-triazin-3-yl)pyridine. Inorganics 2019, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Catellani, C.B.; Carugo, O.; Giusti, M.; Sardone, N. Crystal structure of scandium(III) triflate enneahydrated. Eur. J. Solid State Chem. 1995, 32, 1089–1099. [Google Scholar]
- Starobrat, A.; Jaron, T.; Grochala, W. Synthesis and characterization of a series of mixed-cation borohydrides of scandium: [Cat][Sc(BH4)4], [Cat] = [Me4N], [n-Bu4N], and [Ph4P]. Inorg. Chim. Acta 2015, 437, 70–73. [Google Scholar] [CrossRef]
- Jenter, J.; Meyer, N.; Roesky, P.W.; Thiele, S.K.-H.; Eickering, G.; Scherer, W. Borane and Borohydride Complexes of the Rare-Earth Elements: Synthesis, Structures, and Butadiene Polymerization Catalysis. Chem. Eur. J. 2010, 16, 5472–5480. [Google Scholar] [CrossRef]
- Johnson, N.W. Convex Polyhedra with Regular Phases. Can. J. Math. 1966, 18, 169–200. [Google Scholar] [CrossRef]
- Alvarez, S. Polyhedra in (inorganic) chemistry. Dalton Trans. 2005, 13, 2209–2233. [Google Scholar] [CrossRef]
- Ruiz-Martinez, A.; Casanova, D.; Alvarez, S. Polyhedral structures with an odd number of vertices: Nine-atom clusters and supramolecular architectures. Dalton Trans. 2008, 2583–2591. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. Shape—Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools. University of Barcelona. Available online: https://www.ee.ub.edu/downloads/ (accessed on 20 May 2023).
- Xue, Z.-L.; Cook, T.M. NMR of Inorganic Nuclei. In Comprehensive Coordination Chemistry III; Reedijk, J., Poeppelmeier, K.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Kellö, V.; Sadlej, A.J.; Pyykkö, P. The nuclear quadrupole moment of 45Sc. Chem. Phys. Lett. 2000, 329, 112–118. [Google Scholar] [CrossRef]
- Anderson, T.J.; Neumann, M.A.; Melson, G.A. Coordination chemistry of scandium. V.Crystal and molecular structure of tris(acetylacetonato)scandium(III). Inorg. Chem. 1973, 12, 927–930. [Google Scholar] [CrossRef]
- Streltsova, N.R.; Belskii, V.K.; Bulychev, B.M.; Kireeva, O.K. Crystalline Structure of [ScCl2(15-crown-5)2][CuCl4], a Heterobimetal Complex. Zh. Neorg. Khim. Russ. J. Inorg. Chem. 1992, 37, 1822–1827. [Google Scholar]
- Brown, M.D.; Levason, W.; Murray, D.C.; Popham, M.C.; Reid, G.; Webster, M. Primary and secondary coordination of crown ethers to scandium(III). Synthesis, properties and structures of the reaction products of ScCl3(thf)3, ScCl3·6H2O and Sc(NO3)3·5H2O with crown ethers. Dalton Trans. 2003, 857–865. [Google Scholar] [CrossRef]
- Willey, G.R.; Lakin, M.T.; Alcock, N.W. Crown Ether Complexation of Scandium(III), Yttrium(III) and Lanthanum(III) Halides. Synthesis and Spectroscopic Characterisation of Anhydrous Cationic Metal-Oxacrown Species and Crystal Structure of a Scandium Benzocrown. J. Chem. Soc. Dalton Trans. 1993, 3407–3411. [Google Scholar] [CrossRef]
- Uson, R.; Laguna, A.; Laguna, M.; Briggs, D.A.; Murray, H.H.; Fackler, J.P., Jr. (Tetrahydrothiophene)Gold(I) or Gold(III) Complexes. Inorg. Synth. 1989, 26, 85–91. [Google Scholar]
- Kowala, C.; Swan, J.M. Coordination compounds of Group IB metals. II. Some tertiary phosphine and phosphite complexes of gold(I). Aust. J. Chem. 1966, 19, 547–554. [Google Scholar] [CrossRef]
- Dixon, A.E.; Taylor, J. LXIV.-Substituted isothiohydantoins. J. Chem. Soc. Trans. 1912, 101, 558–561. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, M.; Ikuma, T.; Obara, N.; Togo, H. Synthesis of mesoionic triazoline nucleosides. J. Chem. Soc. Perkin Trans. 1990, 1, 3243–3245. [Google Scholar] [CrossRef]
- Sheldrick, G. SADABS, vers. 2014/5; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Coppens, P. The Evaluation of Absorption and Extinction in Single-Crystal Structure Analysis. Crystallographic Computing; Muksgaard: Copenhagen, Denmark, 1979. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Diamond—Crystal and Molecular Structure Visualization; vers. 4.6.8.; Crystal Impact: Bonn, Germany, 2022. [Google Scholar]













| Au1–S1 | S1–C12 | C12–N12 | C12–N11 | N11–C11 | Au2–S2 | S2–C22 | C22–N22 | C22–N21 | N21–C21 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 2.266(1) | 1.710(4) | 1.305(5) | 1.408(5) | 1.375(5) | 2.270(1) | 1.717(4) | 1.313(5) | 1.398(5) | 1.382(5) |
| 20 | 2.296(3) | 1.73(1) 1 | 1.36(2) 1 | 1.33(2) | 1.22(3) | |||||
| H2L1ethyl [42] | 1.62(2) | 1.35(2) 1 | 1.45(2) | 1.33(2) | 1.65(2) | 1.32(2) | 1.43(2) | 1.35(2) |
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. G. 1 | C2/c | |||||||||||||
| Radii 2 | 1.216 | 1.196 | 1.179 | 1.163 | 1.132 | 1.12 | 1.107 | 1.095 | 1.083 | 1.072 | 1.062 | 1.052 | 1.042 | 1.032 |
| M-N | 2.657(6) | 2.623(6) | 2.589(9) | 2.594(6) | 2.554(8) | 2.54(1) | 2.529(6) | 2.514(3) | 2.49(1) | 2.487(4) | 2.480(6) | 2.444(6) 2.454(3) | 2.45(1) | 2.429(6)– 2.447(6) 3 |
| M-O | 2.510(6) 2.555(5) | 2.525(5) 2.482(5) | 2.471(9) 2.520(9) | 2.502(5) 2.461(5) | 2.465(7) 2.428(7) | 2.453(8) 2.421(9) | 2.445(5) 2.405(5) | 2.435(3) 2.392(3) | 2.378(8) 2.420(7) | 2.417(3) 2.371(4) | 2.372(5) 2.428(5) | 2.378(3) 2.342(3) 2.403(3) | 2.371(9) 2.34(1) | 2.298(5)– 2.391(5) 4 |
| Au-S | 2.267(2) 2.276(3) | 2.274(2) 2.284(2) | 2.278(4) 2.294(4) | 2.290(2) 2.277(2) | 2.774(3) 2.285(3) | 2.280(3) 2.289(4) | 2.276(2) 2.291(2) | 2.278(1) 2.289(1) | 2.279(3) 2.291(4) | 2.281(1) 2.294(2) | 2.295(2) 2.313(2) | 2.286(2) 2.277(2) 2.284(1) | 2.292(4) 2.275(4) | 2.269(2)– 2.289(2) 5 |
| C-S | 1.739(8) 1.74(1) | 1.73(1) 1.738(7) | 1.69(2) 1.74(1) | 1.744(7) 1.742(9) | 1.74(1) 1.75(1) | 1.73(2) 1.74(1) | 1.747(8) 1.74(1) | 1.743(5) 1.74(4) | 1.74(2) 1.74(1) | 1.741(5) 1.748(7) | 1.734(8) 1.747(9) | 1.730(6) 1.747(5) 1.75(1) | 1.76(2) 1.73(2) | 1.729(9)– 1.777(9) 6 |
| S-Au-S | 172.8(1) | 173.9(1) | 173.8(2) | 173.54(9) | 173.9(1) | 174.2(2) | 174.1(1) | 173.97(5) | 174.4(2) | 174.26(7) | 174.4(1) | 176.80(5) 168.91(7) | 174.4(2) | 174.6(1)–177.4(1) 7 |
| Sc (15) | Y (16) | In (17) | Ga (18a) | Ga (18b) | |
|---|---|---|---|---|---|
| S. G. 1 | P21/c | C2/c | P21/c | P21/c | |
| Radii 2 | 0.87 (for C.N. 8) | 1.075 (for C.N. 8) | 0.92 (for C.N. 8) | 0.62 (for C.N. 6) | 0.62 (for C.N. 6) |
| M-N | 2.350(2), 2.350(2), 2.365(2) | 2.50(1), 2.50(1), 2.49(1) | 2.330(3), 2.334(2) | 1.961(4), 1.950(4) | 1.90(2), 1.95(2) |
| M-O | 2.248(2), 2.291(2), 2.221(2), 2.291(2), 2.294(2), 2.316(2) | 2.441(9), 2.337(9), 2.362(8), 2.352(9), 2.281(9), 2.463(9) | 2.354(2), 2.322(2), 2.391(3) | 1.977(4), 1.988(3), 2.010(4), 1.991(4) | 1.98(1), 1.96(1), 1.97(1), 1.97(1) |
| Au-S | 2.2891(6), 2.2838(6), 2.2811(6), 2.2923(6), 2.2915(6), 2.2771(6) | 2.2888(5), 2.302(4), 2.290(5), 2.289(5), 2.294(4), 2.289(4) | 2.287(1), 2.290(1), 2.402(7) 3 | 2.277(2), 2.279(2), 2.2757(1), 2.2670(1) | 2.270(7), 2.268(7), 2.278(7), 2.303(7) |
| C-S | 1.760(2), 1.754(2), 1.750(2), 1.741(2), 1.749(2), 1.736(2) | 1.74(2), 1.73(1), 1.73(2), 1.72(2), 1.73(1), 1.73(2) | 1.761(4), 1.740(4), 1.75(1) 3 | 1.738(6), 1.736(5), 1.738(6), 1.723(5) | 1.71(3), 1.78(2), 1.71(2), 1.77(2) |
| S-Au-S | 176.61(2), 174.15(2), 175.90(2) | 175.6(1), 177.4(2), 1.76.8(1) | 168.44(6), 174.2(2) 3 | 166.70(5), 167.29(5) | 168.2(2), 1.669(2) |
| Capped Cube r-CCU-9 1 | Hula Hoop HH-9 | Muffin MFF-9 | Tridiminished Icosahedron TDIC-9 | Tricapped Trigonal Prism s-TCTPR-9 1 | Capped Square Antiprism r-CSAPR-9 1 | |
|---|---|---|---|---|---|---|
| La (1) | 8.675 | 11.876 | 2.743 | 10.652 | 2.230 | 2.416 |
| Ce(2) | 8.709 | 11.890 | 2.627 | 10.551 | 2.086 | 2.309 |
| Pr (3) | 8.647 | 11.892 | 2.562 | 10.546 | 1.949 | 2.206 |
| Nd (4) | 8.813 | 11.873 | 2.415 | 10.888 | 1.769 | 2.027 |
| Sm (5) | 8.900 | 11.915 | 2.275 | 11.101 | 1.567 | 1.857 |
| Eu (6) | 8.932 | 11.957 | 2.210 | 11.033 | 1.455 | 1.779 |
| Gd (7) | 8.967 | 11.962 | 2.181 | 11.099 | 1.390 | 1.727 |
| Tb (8) | 9.019 | 11.977 | 2.131 | 11.220 | 1.309 | 1.658 |
| Dy (9) | 9.053 | 12.051 | 2.061 | 11.263 | 1.186 | 1.570 |
| Y (10) | 9.134 | 11.881 | 1.835 | 12.749 | 1.203 | 1.157 |
| Ho (11) | 9.080 | 12.081 | 2.041 | 11.240 | 1.125 | 1.531 |
| Er (12) | 9.127 | 12.140 | 1.986 | 11.133 | 0.993 | 1.450 |
| Tm (13) | 9.016 | 11.993 | 1.847 | 12.723 | 0.978 | 1.311 |
| Yb (14) | 9.194 | 12.146 | 1.963 | 11.452 | 1.029 | 1.448 |
| Lu (15) | 9.265 | 11.952 | 1.876 | 12.370 | 0.926 | 1.228 |
| In (16) | 9.790 | 12.708 | 1.503 | 12.972 | 0.406 | 0.910 |
| Sc (17) | 9.707 | 12.229 | 1.633 | 12.711 | 0.614 | 0.986 |
| [Zn⊂{Au2(L1ethyl)2}] (21) | ||||||||||||
| Zn–N1 | 2.035(3) | Zn–N2 | 2.050(3) | Zn–N11 | 2.221(3) | Zn–O21 | 2.154(3) | |||||
| Zn–O31 | 2.120(2) | Zn-O41 | 2.181(2) | Au1-S1 | 2.283(1) | Au1–S3 | 2.291(1) | |||||
| Au2–S2 | 2.289(1) | Au2-S4 | 2.289(1) | N1–Zn–N2 | 174.8(1) | N1–Zn–O21 | 77.1(1) | |||||
| N1–Zn–O31 | 108.6(1) | N1–Zn–O21 | 99.4(1) | N1–Zn–N11 | 75.0(1) | N2–Zn–O21 | 101.8(2) | |||||
| N2–Zn–O31 | 76.5(1) | N2–Zn–O41 | 75.5(1) | N2–Zn–N11 | 105.9(1) | O21–Zn–N11 | 152.0(1) | |||||
| O21–Zn–O41 | 90.5(1) | O31–Zn–N11 | 97.3(1) | O31–Zn–O21 | 93.8(1) | O31–Zn–O41 | 151.87(9) | |||||
| O41–Zn–N11 | 92.7(1) | S1–Au1–S3 | 173.24(4) | S2–Au2–S4 | 166.98(4) | |||||||
| [Sc(H2O)2{Au(L1ethyl)2}] (22) | ||||||||||||
| Sc–O1 | 2.224(6)/2.147(7) | Sc–O2 | 2.171(6)/2.177(6) | Sc-N1 | 2.288(6)/2.322(8) | |||||||
| Sc–O11 | 2.198(6)/2.269(6) | Sc–O21 | 2.190(6)/2.153(6) | Sc–N2 | 2.304(6)/2.339(7) | |||||||
| Sc–O31 | 2.210(6)/2.197(7) | Sc–O41 | 2.209(6)/2.252(7) | Au1–S1 | 2.275(4)/2.284(3) | |||||||
| Au2–S2 | 2.280(3)/2.283(3) | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sucena, S.F.; Demirer, T.I.; Baitullina, A.; Hagenbach, A.; Grewe, J.; Spreckelmeyer, S.; März, J.; Barkleit, A.; Maia, P.I.d.S.; Nguyen, H.H.; et al. Gold-Based Coronands as Hosts for M3+ Metal Ions: Ring Size Matters. Molecules 2023, 28, 5421. https://doi.org/10.3390/molecules28145421
Sucena SF, Demirer TI, Baitullina A, Hagenbach A, Grewe J, Spreckelmeyer S, März J, Barkleit A, Maia PIdS, Nguyen HH, et al. Gold-Based Coronands as Hosts for M3+ Metal Ions: Ring Size Matters. Molecules. 2023; 28(14):5421. https://doi.org/10.3390/molecules28145421
Chicago/Turabian StyleSucena, Suelen Ferreira, Türkan Ilgin Demirer, Anna Baitullina, Adelheid Hagenbach, Jacqueline Grewe, Sarah Spreckelmeyer, Juliane März, Astrid Barkleit, Pedro Ivo da Silva Maia, Hung Huy Nguyen, and et al. 2023. "Gold-Based Coronands as Hosts for M3+ Metal Ions: Ring Size Matters" Molecules 28, no. 14: 5421. https://doi.org/10.3390/molecules28145421