Modification of the Surface Composition of PTB7-Th: ITIC Blend Using an Additive
Abstract
:1. Introduction
2. Results and Discussion
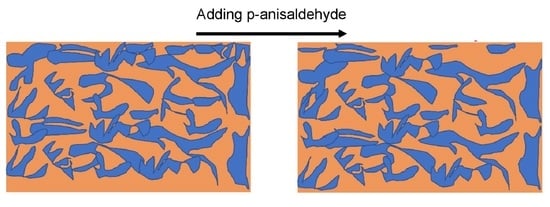
2.1. Device Performance
2.2. AFM Results
2.3. NICISS Results
3. Experimental Procedure: Material and Device Fabrication
3.1. Materials
3.2. Device Fabrication
3.3. Characterization
3.3.1. Device Performance
3.3.2. Neutral Impact Collision Ion Spectroscopy (NICISS)
3.3.3. Atomic Force Microscope (AFM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability Statement
References
- Cui, Y.; Xu, Y.; Yao, H.; Bi, P.; Hong, L.; Zhang, J.; Zu, Y.; Zhang, T.; Qin, J.; Ren, J. Single-junction organic photovoltaic cell with 19% efficiency. Adv. Mater. 2021, 33, 2102420. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Qi, F.; Peng, Z.; Lin, F.R.; Jiang, K.; Zhong, C.; Kaminsky, W.; Guan, Z.; Lee, C.S.; Marks, T.J. Achieving 19% Power Conversion Efficiency in Planar-Mixed Heterojunction Organic Solar Cells Using a Pseudo-Symmetric Electron Acceptor. Adv. Mater. 2022, 34, 2202089. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Tajima, K. Organic Planar Heterojunctions: From Models for Interfaces in Bulk Heterojunctions to High-Performance Solar Cells. Adv. Mater. 2017, 29, 1603269. [Google Scholar] [CrossRef]
- Richter, L.J.; DeLongchamp, D.M.; Amassian, A. Morphology development in solution-processed functional organic blend films: An in situ viewpoint. Chem. Rev. 2017, 117, 6332–6366. [Google Scholar] [CrossRef]
- Chen, L.M.; Hong, Z.; Li, G.; Yang, Y. Recent progress in polymer solar cells: Manipulation of polymer: Fullerene morphology and the formation of efficient inverted polymer solar cells. Adv. Mater. 2009, 21, 1434–1449. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Ye, L.; Collins, B.A.; Jiao, X.; Zhao, J.; Yan, H.; Ade, H. Miscibility–function relations in organic solar cells: Significance of optimal miscibility in relation to percolation. Adv. Energy Mater. 2018, 8, 1703058. [Google Scholar] [CrossRef]
- Han, X.; Zhou, S.J.; Tan, Y.Z.; Wu, X.; Gao, F.; Liao, Z.J.; Huang, R.B.; Feng, Y.Q.; Lu, X.; Xie, S.Y. Crystal Structures of Saturn-Like C50Cl10 and Pineapple-Shaped C64Cl4: Geometric Implications of Double-and Triple-Pentagon-Fused Chlorofullerenes. Angew. Chem. Int. Ed. 2008, 47, 5340–5343. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Zhang, W.; Meng, X.; Ma, W.; Yartsev, A.; Inganas, O.; Andersson, M.R.; Janssen, R.A.; Wang, E. High performance all-polymer solar cells by synergistic effects of fine-tuned crystallinity and solvent annealing. J. Am. Chem. Soc. 2016, 138, 10935–10944. [Google Scholar] [CrossRef]
- Peet, J.; Kim, J.Y.; Coates, N.E.; Ma, W.L.; Moses, D.; Heeger, A.J.; Bazan, G.C. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 2007, 6, 497–500. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Hou, J.; Zhang, S.; Liang, Y.; Yang, G.; Yang, Y.; Yu, L.; Wu, Y.; Li, G. Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat. Photonics 2009, 3, 649–653. [Google Scholar] [CrossRef]
- Moulé, A.J.; Meerholz, K. Controlling morphology in polymer–fullerene mixtures. Adv. Mater. 2008, 20, 240–245. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, X.; Ma, X.; Zhou, H.; Li, X.; Jeong, S.Y.; Woo, H.Y.; Zhou, Z.; Sun, Q.; Zhang, F. Achieving 15.81% and 15.29% efficiency of all-polymer solar cells based on layer-by-layer and bulk heterojunction structures. J. Mater. Chem. A 2022, 10, 13492–13499. [Google Scholar] [CrossRef]
- Shi, G.; Yuan, J.; Huang, X.; Lu, Y.; Liu, Z.; Peng, J.; Ding, G.; Shi, S.; Sun, J.; Lu, K. Combinative effect of additive and thermal annealing processes delivers high efficiency all-polymer solar cells. J. Phys. Chem. C 2015, 119, 25298–25306. [Google Scholar] [CrossRef]
- Gurney, R.S.; Li, W.; Yan, Y.; Liu, D.; Pearson, A.J.; Wang, T. Morphology and efficiency enhancements of PTB7-Th: ITIC nonfullerene organic solar cells processed via solvent vapor annealing. J. Energy Chem. 2019, 37, 148–156. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; He, Z.; Wu, H.; Cao, Y. Influence of the acceptor crystallinity on the open-circuit voltage in PTB7-Th: ITIC organic solar cells. J. Mater. Chem. C 2019, 7, 14861–14866. [Google Scholar] [CrossRef]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing additives for improved efficiency from bulk heterojunction solar cells. J. Am. Chem. Soc. 2008, 130, 3619–3623. [Google Scholar] [CrossRef]
- Love, J.A.; Chou, S.-H.; Huang, Y.; Bazan, G.C.; Nguyen, T.-Q. Effects of solvent additive on “s-shaped” curves in solution-processed small molecule solar cells. Beilstein J. Org. Chem. 2016, 12, 2543–2555. [Google Scholar] [CrossRef]
- Collins, B.A.; Li, Z.; Tumbleston, J.R.; Gann, E.; McNeill, C.R.; Ade, H. Absolute measurement of domain composition and nanoscale size distribution explains performance in PTB7: PC71BM solar cells. Adv. Energy Mater. 2013, 3, 65–74. [Google Scholar] [CrossRef]
- Van Franeker, J.J.; Turbiez, M.; Li, W.; Wienk, M.M.; Janssen, R.A. A real-time study of the benefits of co-solvents in polymer solar cell processing. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Proctor, C.M.; Tumbleston, J.R.; Bazan, G.C.; Nguyen, T.Q.; Ade, H. Importance of Domain Purity and Molecular Packing in Efficient Solution-Processed Small-Molecule Solar Cells. Adv. Mater. 2015, 27, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gasparini, N.; Ye, L.; Yao, H.; Hou, J.; Ade, H.; Baran, D. Controlling blend morphology for ultrahigh current density in nonfullerene acceptor-based organic solar cells. ACS Energy Lett. 2018, 3, 669–676. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, Y.; Wang, X.; Wu, Y.; Feng, G.; Li, C.; Ma, W.; Li, W. Non-fullerene organic solar cells based on diketopyrrolopyrrole polymers as electron donors and ITIC as an electron acceptor. Phys. Chem. Chem. Phys. 2017, 19, 8069–8075. [Google Scholar] [CrossRef]
- Song, X.; Gasparini, N.; Baran, D. The Influence of Solvent Additive on Polymer Solar Cells Employing Fullerene and Non-Fullerene Acceptors. Adv. Electron. Mater. 2018, 4, 1700358. [Google Scholar] [CrossRef]
- Zhao, W.; Qian, D.; Zhang, S.; Li, S.; Inganäs, O.; Gao, F.; Hou, J. Fullerene-free polymer solar cells with over 11% efficiency and excellent thermal stability. Adv. Mater. 2016, 28, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Jiao, X.; Zhao, W.; Zhang, S.; Yao, H.; Li, S.; Ade, H.; Hou, J. Manipulation of domain purity and orientational ordering in high performance all-polymer solar cells. Chem. Mater. 2016, 28, 6178–6185. [Google Scholar] [CrossRef]
- Cheng, P.; Lin, Y.; Zawacka, N.K.; Andersen, T.R.; Liu, W.; Bundgaard, E.; Jørgensen, M.; Chen, H.; Krebs, F.C.; Zhan, X. Comparison of additive amount used in spin-coated and roll-coated organic solar cells. J. Mater. Chem. A 2014, 2, 19542–19549. [Google Scholar] [CrossRef]
- Zhao, F.; Dai, S.; Wu, Y.; Zhang, Q.; Wang, J.; Jiang, L.; Ling, Q.; Wei, Z.; Ma, W.; You, W. Single-junction binary-blend nonfullerene polymer solar cells with 12.1% efficiency. Adv. Mater. 2017, 29, 1700144. [Google Scholar] [CrossRef]
- Li, Z.; Dai, S.; Xin, J.; Zhang, L.; Wu, Y.; Rech, J.; Zhao, F.; Li, T.; Liu, K.; Liu, Q. Enhancing the performance of the electron acceptor ITIC-Th via tailoring its end groups. Mater. Chem. Front. 2018, 2, 537–543. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, F.; He, Q.; Huo, L.; Wu, Y.; Parker, T.C.; Ma, W.; Sun, Y.; Wang, C.; Zhu, D. High-performance electron acceptor with thienyl side chains for organic photovoltaics. J. Am. Chem. Soc. 2016, 138, 4955–4961. [Google Scholar] [CrossRef]
- Tremolet de Villers, B.J.; O’Hara, K.A.; Ostrowski, D.P.; Biddle, P.H.; Shaheen, S.E.; Chabinyc, M.L.; Olson, D.C.; Kopidakis, N. Removal of residual diiodooctane improves photostability of high-performance organic solar cell polymers. Chem. Mater. 2016, 28, 876–884. [Google Scholar] [CrossRef]
- Weu, A.; Kress, J.A.; Paulus, F.; Becker-Koch, D.; Lami, V.; Bakulin, A.A.; Vaynzof, Y. Oxygen-induced doping as a degradation mechanism in highly efficient organic solar cells. ACS Appl. Energy Mater. 2019, 2, 1943–1950. [Google Scholar] [CrossRef]
- Holliday, S.; Luscombe, C.K. Low boiling point solvent additives for improved photooxidative stability in organic photovoltaics. Adv. Electron. Mater. 2018, 4, 1700416. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Z.; Dong, S.; Zheng, N.; Ying, L.; Jiang, X.F.; Liu, F.; Huang, F.; Cao, Y. A novel naphtho [1, 2-c: 5, 6-c′] bis ([1,2,5] thiadiazole)-based narrow-bandgap π-conjugated polymer with power conversion efficiency over 10%. Adv. Mater. 2016, 28, 9811–9818. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.W.; Yan, B.; Li, R.; Li, E.Q.; Zhao, K.; Anjum, D.H.; Alvarez, S.; Gassaway, R.; Biocca, A.; Thoroddsen, S.T. Spin-cast bulk heterojunction solar cells: A dynamical investigation. Adv. Mater. 2013, 25, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yuan, Y.; Yang, B.; VanDerslice, J.; Chen, J.; Dyck, O.; Duscher, G.; Huang, J. Universal formation of compositionally graded bulk heterojunction for efficiency enhancement in organic photovoltaics. Adv. Mater. 2014, 26, 3068–3075. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kitazawa, D.; Tsukamoto, J.; Shibamori, T.; Seki, H.; Nakagawa, Y. Composition depth profile analysis of bulk heterojunction layer by time-of-flight secondary ion mass spectrometry with gradient shaving preparation. Thin Solid Film. 2010, 518, 2115–2118. [Google Scholar] [CrossRef]
- Germack, D.S.; Chan, C.K.; Kline, R.J.; Fischer, D.A.; Gundlach, D.J.; Toney, M.F.; Richter, L.J.; DeLongchamp, D.M. Interfacial segregation in polymer/fullerene blend films for photovoltaic devices. Macromolecules 2010, 43, 3828–3836. [Google Scholar] [CrossRef]
- Jasieniak, J.J.; Treat, N.D.; McNeill, C.R.; de Villers, B.J.T.; Della Gaspera, E.; Chabinyc, M.L. Interfacial characteristics of efficient bulk heterojunction solar cells fabricated on moox anode interlayers. Adv. Mater. 2016, 28, 3944–3951. [Google Scholar] [CrossRef]
- Chen, M.; Liu, D.; Li, W.; Gurney, R.S.; Li, D.; Cai, J.; Spooner, E.L.; Kilbride, R.C.; McGettrick, J.D.; Watson, T.M. Influences of non-fullerene acceptor fluorination on three-dimensional morphology and photovoltaic properties of organic solar cells. ACS Appl. Mater. Interfaces 2019, 11, 26194–26203. [Google Scholar] [CrossRef]
- Huang, S.; Huai, Z.; Ren, J.; Sun, Y.; Zhao, X.; Wang, L.; Kong, W.; Fu, G.; Yang, S. Improved Morphology and Interfacial Contact of PBDB-T: N2200-Based All-Polymer Solar Cells by Using the Solvent Additive p-Anisaldehyde. ACS Appl. Energy Mater. 2019, 3, 358–365. [Google Scholar] [CrossRef]
- Sprau, C.; Buss, F.; Wagner, M.; Landerer, D.; Koppitz, M.; Schulz, A.; Bahro, D.; Schabel, W.; Scharfer, P.; Colsmann, A. Highly efficient polymer solar cells cast from non-halogenated xylene/anisaldehyde solution. Energy Environ. Sci. 2015, 8, 2744–2752. [Google Scholar] [CrossRef]
- Ho, C.H.Y.; Dong, Q.; Yin, H.; Leung, W.W.K.; Yang, Q.; Lee, H.K.H.; Tsang, S.W.; So, S.K. Impact of Solvent Additive on Carrier Transport in Polymer:Fullerene Bulk Heterojunction Photovoltaic Cells. Adv. Mater. Interfaces 2015, 2, 1500166. [Google Scholar] [CrossRef]
- Sharenko, A.; Gehrig, D.; Laquai, F.; Nguyen, T.-Q. The Effect of Solvent Additive on the Charge Generation and Photovoltaic Performance of a Solution-Processed Small Molecule:Perylene Diimide Bulk Heterojunction Solar Cell. Chem. Mater. 2014, 26, 4109–4118. [Google Scholar] [CrossRef]
- Perez, L.A.; Rogers, J.T.; Brady, M.A.; Sun, Y.; Welch, G.C.; Schmidt, K.; Toney, M.F.; Jinnai, H.; Heeger, A.J.; Chabinyc, M.L.; et al. The Role of Solvent Additive Processing in High Performance Small Molecule Solar Cells. Chem. Mater. 2014, 26, 6531–6541. [Google Scholar] [CrossRef]
- Jang, Y.; Ju Cho, Y.; Kim, M.; Seok, J.; Ahn, H.; Kim, K. Formation of Thermally Stable Bulk Heterojunction by Reducing the Polymer and Fullerene Intermixing. Sci. Rep. 2017, 7, 9690. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Y.; Yu, T.; Zhang, Q.; Masse, J.-P.; Yang, Y.; Izquierdo, R.; Sun, B.; Ma, D. Unveiling photovoltaic performance enhancement mechanism of polymer solar cells via synergistic effect of binary solvent additives. Sol. RRL 2020, 4, 2000239. [Google Scholar] [CrossRef]
- Dehoff, R.; Duty, C.; Peter, W.; Yamamoto, Y.; Chen, W.; Blue, C.; Tallman, C. Case study: Additive manufacturing of aerospace brackets. Adv. Mater. Processes 2013, 171, 19–23. [Google Scholar]
- Schmerl, N.; Andersson, G. A layered structure at the surface of P3HT/PCBM blends. Phys. Chem. Chem. Phys. 2011, 13, 14993–15002. [Google Scholar] [CrossRef]
- Tan, H.L.; Krebs, T.; Andersson, G.; Neff, D.; Norton, M.; Morgner, H.; Van Patten, P.G. Internal structure of polyelectrolyte multilayers probed via neutral impact collision ion scattering spectroscopy. Langmuir 2005, 21, 2598–2604. [Google Scholar] [CrossRef]
- Andersson, G.; Morgner, H. Investigations on solutions of tetrabutylonium salts in formamide with NICISS and ICISS: Concentration depth profiles and composition of the outermost layer. Surf. Sci. 2000, 445, 89–99. [Google Scholar] [CrossRef]
- Chen, H.; Hsiao, Y.C.; Hu, B.; Dadmun, M. Tuning the morphology and performance of low bandgap polymer: Fullerene heterojunctions via solvent annealing in selective solvents. Adv. Funct. Mater. 2014, 24, 5129–5136. [Google Scholar] [CrossRef]
- van Franeker, J.J.; Heintges, G.H.; Schaefer, C.; Portale, G.; Li, W.; Wienk, M.M.; van der Schoot, P.; Janssen, R.A. Polymer solar cells: Solubility controls fiber network formation. J. Am. Chem. Soc. 2015, 137, 11783–11794. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jin, Y.; Dong, S.; Zheng, W.; Yang, J.; Liu, A.; Liu, F.; Jiang, Y.; Russell, T.P.; Zhang, F. Printed nonfullerene organic solar cells with the highest efficiency of 9.5%. Adv. Energy Mater. 2018, 8, 1701942. [Google Scholar] [CrossRef]
- Wang, C.; Xu, X.; Zhang, W.; Dkhil, S.B.; Meng, X.; Liu, X.; Margeat, O.; Yartsev, A.; Ma, W.; Ackermann, J. Ternary organic solar cells with enhanced open circuit voltage. Nano Energy 2017, 37, 24–31. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO film as an electron transport layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.; Ridings, C. Ion Scattering Studies of Molecular Structure at Liquid Surfaces with Applications in Industrial and Biological Systems. Chem. Rev. 2014, 114, 8361–8387. [Google Scholar] [CrossRef]
- Andersson, G.; Morgner, H.; Pohl, H. Energy-loss straggling of helium projectiles at low kinetic energies: Deconvolution of concentration depth profiles of inorganic salt solutes in aqueous solutions. Phys. Rev. A 2008, 78, 032904. [Google Scholar] [CrossRef]
- Andersson, G.; Morgner, H. Impact collision ion scattering spectroscopy (ICISS) and neutral impact collision ion scattering spectroscopy (NICISS) at surfaces of organic liquids. Surf. Sci. 1998, 405, 138–151. [Google Scholar] [CrossRef]
- Wang, C.; Kan, A.; Liu, Z.; Zhang, G.; Lin, X.; Fu, H. Using neutral impact collision ion scattering spectroscopy and angular resolved X-ray photoelectron spectroscopy to analyze surface structure of surfactant solutions. Colloid Polym. Sci. 2015, 293, 1655–1666. [Google Scholar] [CrossRef]
- Andersson, G.; Krebs, T.; Morgner, H. Activity of surface active substances determined from their surface excess. Phys. Chem. Chem. Phys. 2005, 7, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, G.; Morgner, H. Determining the stopping power of low energy helium in alkanethiolates with Neutral Impact Collision Ion Scattering Spectroscopy (NICISS). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1999, 155, 357–368. [Google Scholar] [CrossRef]
- Zhao, X.; Nathanson, G.M.; Andersson, G.G. Experimental Depth Profiles of Surfactants, Ions, and Solvent at the Angstrom Scale: Studies of Cationic and Anionic Surfactants and Their Salting Out. J. Phys. Chem. B 2020, 124, 2218–2229. [Google Scholar] [CrossRef] [PubMed]





| Device | JSC (mA cm−2) | VOC (V) | FF | PCE (%) |
|---|---|---|---|---|
| PTB7-Th: ITIC + 0% AA | 15.55 ± 0.16 | 0.81 ± 0.01 | 0.56 ± 0.01 | 7.03 ± 0.13 |
| PTB7-Th: ITIC + 2% AA | 16.47 ± 0.18 | 0.80 ± 0.01 | 0.62 ± 0.01 | 8.20 ± 0.21 |
| Device | Surface Roughness (nm) (AFM) | Relative S Concentration (NICISS) |
|---|---|---|
| PTB7-Th: ITIC + 0% AA | 1.1 ± 0.1 | 0.78 ± 0.1 |
| PTB7-Th: ITIC + 2% AA | 5.4 ± 0.1 | 0.90 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, A.R.; Kirk, B.P.; Kocak, G.; Andersson, M.R.; Andersson, G.G. Modification of the Surface Composition of PTB7-Th: ITIC Blend Using an Additive. Molecules 2022, 27, 6358. https://doi.org/10.3390/molecules27196358
Alghamdi AR, Kirk BP, Kocak G, Andersson MR, Andersson GG. Modification of the Surface Composition of PTB7-Th: ITIC Blend Using an Additive. Molecules. 2022; 27(19):6358. https://doi.org/10.3390/molecules27196358
Chicago/Turabian StyleAlghamdi, Amira R., Bradley P. Kirk, Guler Kocak, Mats R. Andersson, and Gunther G. Andersson. 2022. "Modification of the Surface Composition of PTB7-Th: ITIC Blend Using an Additive" Molecules 27, no. 19: 6358. https://doi.org/10.3390/molecules27196358