Emerging Management Approach for the Adverse Events of Immunotherapy of Cancer
Abstract
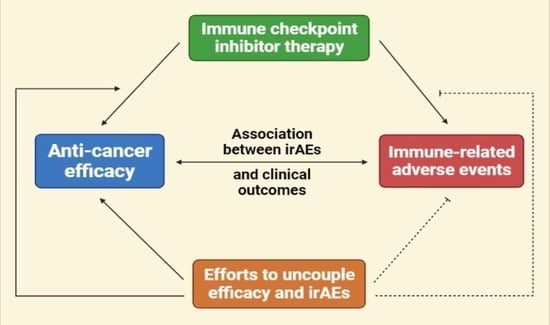
:1. Introduction
2. Overview of Immune Checkpoint Inhibitor
3. Atypical Patterns of Responses
3.1. Delayed Response
3.2. Hyper Progression
4. Immune Checkpoints in Cancer
4.1. Immune Checkpoint Inhibitor (ICI) Approved for dMMR-MSI-H Cancers
4.2. CLTA4 Checkpoint Inhibition and Therapy
4.3. Inhibition of the PD-1 and/or PD-L1 Checkpoints, as Well as Treatment
| Target | Drug | Condition | Treatment Regimen | Treatment in Control Group | Objective Response Rate % | Reference |
|---|---|---|---|---|---|---|
| Programmed cell death protein 1 (PD-1) signaling | Nivolumab (IgG4a) | Melanoma (stage III/IV) | 3 mg/kg/2 week | Combination therapy | 43.7 | [60] |
| Renal cell carcinoma (metastatic) | 3 mg/kg/2 weeks | 10 mg/day Everolimus | 25 (4% control) | [61] | ||
| Hodgkin’s lymphoma (relapsed/refractory) | 3 mg/kg/2 weeks | n/a | 87 | [62] | ||
| Squamous-cell carcinoma of the head and neck (recurrent) | 3 mg/kg/2 weeks | Single-agent systemic therapy (methotrexate, docetaxel, or cetuximab) | 13.3 (5.8% control) | [63] | ||
| Ovarian cancer (platinum-resistant) | 1 or 3 mg/kg/2 weeks | n/a | 15 | [64] | ||
| Pembrolizumab (IgG4a) | Melanoma (stage III/IV) | 10 mg/2 weeks or 3 weeks | (vs. ipilimumab) | 33.7–32.9 | [65] | |
| Merkel cell carcinoma | 2 mg/kg/3 weeks | n/a | 56 | [66] | ||
| Progressive metastatic colorectal cancer | 10 mg/kg/every 2 weeks | n/a | 40/0 | [67] | ||
| Pidilizumab (IgG1) | B cell lymphoma (after autologous stem cell transfer | 1.5 mg/42 days | n/a | 51 | [68] | |
| Follicular lymphoma (relapsed) | 3 mg/kg/4 weeks (+rituximab) | n/a | 66 | [69] | ||
| T-lymphocyteassociated protein 4 (CTLA-4) signaling | CTLA-4Ipilimumab (IgG1) | Melanoma (stage III/IV) | 10 mg/kg plus decarbazine | Decarbazine alone | 15.2 (10.3% control) | [70] |
| 3 mg/kg/3 weeks | (vs. Pembrolizumab) | 11.9 | [56] | |||
| 3 mg/kg/3 weeks | (vs. combination with nivolumab) | 19 | [60] | |||
| Tremelimumab (IgG2) | Melanoma (stage III/IV) | 15 mg/kg/90 days | Chemotherapy (temozolomide or dacarbazin) | 10.7 (9.8% control) | [71] | |
| Combination therapy | Nivolumab + Ipilimumab | Melanoma (stage III/IV) | 3 mg/kg/2 weeks Nivolumab 3 mg/kg/3 weeks Ipilimumab | (vs. single) | 57.6 | [60] |
| Non-small cell lung cancer | Nivo + Ipi: 1 + 3 or 3 + 1 mg/ml | Nivolumab alone | 23/19 (10% control) | [72] |
5. Immune Related Adverse Event Patterns
6. Mechanisms Underlying irAEs (Immune Related Adverse Events)

7. Main Features of irAEs
7.1. Diversity
7.2. Hysteresis
7.3. Unpredictability
8. Diagnosis and Management of irAEs
8.1. Dermatological: Rash and Pruritus
8.2. Gastrointestinal: Diarrhea and Colitis
8.3. Hepatotoxic: Hepatitis
8.4. Endocrine: Hypophysitis and Thyropathy
8.5. Respiratory: Pneumonitis
8.6. CAR-T Induced: CRS and SNT
8.7. Rheumatic irAE
8.8. Non-Rheumatic irAEs
8.9. Tumor Response to irAE Treatment
9. Cancer Immunology and Cancer Immunotherapy Advances
10. Blockade of Immune Checkpoints in Cancer Patients
11. Future Direction
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whiteside, T.L. Tumor-derived exosomes and their role in tumor-induced immune suppression. Vaccines 2016, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Santomasso, B.D.; Nastoupil, L.J.; Adkins, S.; Lacchetti, C.; Schneider, B.J.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J. Clin. Oncol. 2021, 39, 3978–3992. [Google Scholar] [CrossRef]
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Saini, S.; Prabhakar, B.S. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. In , , 2020; pp 29–35. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–35. [Google Scholar]
- Elia, G.; Ferrari, S.M.; Galdiero, M.R.; Ragusa, F.; Paparo, S.R.; Ruffilli, I.; Varricchi, G.; Fallahi, P.; Antonelli, A. New insight in endocrine-related adverse events associated to immune checkpoint blockade. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101370. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, S.C.; Pisetsky, D.S. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology 2019, 58, vii59–vii67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savoia, P.; Astrua, C.; Fava, P. Ipilimumab (Anti-Ctla-4 Mab) in the treatment of metastatic melanoma: Effectiveness and toxicity management. Hum. Vaccines Immunother. 2016, 12, 1092–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauf, A.; Abu-Izneid, T.; Khalil, A.A.; Imran, M.; Shah, Z.A.; Emran, T.B.; Mitra, S.; Khan, Z.; Alhumaydhi, F.A.; Aljohani, A.S.; Khan, I. Berberine as a potential anticancer agent: A comprehensive review. Molecules 2021, 26, 7368. [Google Scholar] [CrossRef] [PubMed]
- Dudnik, E.; Bshara, E.; Grubstein, A.; Fridel, L.; Shochat, T.; Roisman, L.C.; Ilouze, M.; Rozenblum, A.B.; Geva, S.; Zer, A.; et al. Rare targetable drivers (RTDs) in non-small cell lung cancer (NSCLC): Outcomes with immune check-point inhibitors (ICPi). Lung Cancer 2018, 124, 117–124. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bibi, S.; Rahaman, M.S.; Rahman, F.; Islam, F.; Khan, M.S.; Hasan, M.M.; Parvez, A.; Hossain, M.A.; Maeesa, S.K.; et al. Pharmacotherapy, Natural therapeutics and nutraceuticals for lung diseases: Traditional significance, phytochemistry, and pharmacology. Biomed. Pharmacother. 2022, 150, 113041. [Google Scholar] [CrossRef]
- Flynn, M.J.; Sayed, A.A.; Sharma, R.; Siddique, A.; Pinato, D.J. Challenges and opportunities in the clinical development of immune checkpoint inhibitors for hepatocellular carcinoma. Hepatology 2019, 69, 2258–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolli, S.S.; Gabros, S.D.; Pona, A.; Cline, A.; Feldman, S.R. Tildrakizumab: A Review of Phase II and III Clinical Trials. Ann. Pharmacother. 2019, 53, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Thomas, X.; Paubelle, E. Tisagenlecleucel-T for the treatment of acute lymphocytic leukemia. Expert Opin. Biol. Ther. 2018, 18, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Zhong, J.F.; Zhang, C. CAR-T cells and allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia. Immunotherapy 2017, 9, 1115–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Razak, A.; Bedard, P.; Siu, L.; Hansen, A. A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann. Oncol. 2015, 26, 1824–1829. [Google Scholar] [CrossRef] [Green Version]
- Gust, J.; Taraseviciute, A.; Turtle, C.J. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs 2018, 32, 1091–1101. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; ElHalawani, H.; Fouad, M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: A meta-analysis. Future Oncol. 2016, 12, 413–425. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1714. [Google Scholar] [CrossRef]
- Pallag, A.; Rosca, E.; Tit, D.M.; Mutiu, G.; Bungau, S.G.; Pop, O.L. Monitoring the effects of treatment in colon cancer cells using immunohistochemical and histoenzymatic techniques. Rom. J. Morphol. Embriol. 2015, 56, 1103–1109. [Google Scholar]
- Das, S.; Ciombor, K.K.; Haraldsdottir, S.; Pumpalova, Y.; Sahin, I.H.; Pineda, G.; Shyr, Y.; Lin, E.; Hsu, C.Y.; Chu, S.K.; et al. Immune-Related Adverse Events and Immune Checkpoint Inhibitor Efficacy in Patients with Gastrointestinal Cancer with Food and Drug Administration-Approved Indications for Immunotherapy. Oncologist 2020, 25, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Alissafi, T.; Hatzioannou, A.; Legaki, A.; Varveri, A.; Verginis, P. Balancing cancer immunotherapy and immune-related adverse events: The emerging role of regulatory T cells. J. Autoimmun. 2019, 104, 102310. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [Green Version]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Frontera, O.A.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef]
- Zell, J.A.; Cinar, P.; Mobasher, M.; Ziogas, A.; Meyskens Jr, F.L.; Anton-Culver, H. Survival for patients with invasive cutaneous melanoma among ethnic groups: The effects of socioeconomic status and treatment. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 66–675. [Google Scholar] [CrossRef] [Green Version]
- Dwary, A.D.; Master, S.; Patel, A.; Cole, C.; Mansour, R.; Mills, G.; Koshy, N.; Peddi, P.; Burton, G.; Hammoud, D.; et al. Excellent response to chemotherapy post immunotherapy. Oncotarget 2017, 8, 91795. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, J.V.; Paludo, J.; McWilliams, R.R.; Zhang, H.; Li, Y.; Kumar, A.B.; Failing, J.; Kottschade, L.A.; Block, M.S.; Markovic, S.N.; et al. Chemo-immunotherapy combination after PD-1 inhibitor failure improves clinical outcomes in metastatic melanoma patients. Melanoma Res. 2020, 30, 364. [Google Scholar] [CrossRef]
- Xia, C.Y.; Wang, D.Y.; Mason, R.; Smith, J.L.; McKean, M.A.; Lo, S.; Guminski, A.D.; Long, G.V.; Carlino, M.S.; Atkinson, V. Activity of targeted therapy after failure of first-line immunotherapy in BRAF-mutant metastatic melanoma. Am. Soc. Clin.Oncol. 2018, 36, 9532. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N. a.; Del Rincon, S.; Papneja, N.; Miller, W.J. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27 (Suppl. S2), 87–97. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butts, C.; Socinski, M.A.; Mitchell, P.L.; Thatcher, N.; Havel, L.; Krzakowski, M.; Nawrocki, S.; Ciuleanu, T.-E.; Bosquée, L.; Trigo, J.M. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, L.H.; Calabrese, C.; Cappelli, L.C. Rheumatic immune-related adverse events from cancer immunotherapy. Nat. Rev. Rheumatol. 2018, 14, 569–579. [Google Scholar] [CrossRef]
- Zhao, F. Surrogate End Points and Their Validation in Oncology Clinical Trials. J. Clin. Oncol. 2016, 34, 1436–1437. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Ko, J.S. The immunology of melanoma. Clin. Lab. Med. 2017, 37, 449–471. [Google Scholar] [CrossRef]
- Koch, M.; Beckhove, P.; op den Winkel, J.; Autenrieth, D.; Wagner, P.; Nummer, D.; Specht, S.; Antolovic, D.; Galindo, L.; Schmitz-Winnenthal, F.H.; et al. Tumor infiltrating T lymphocytes in colorectal cancer: Tumor-selective activation and cytotoxic activity in situ. Ann. Surg. 2006, 244, 986. [Google Scholar] [CrossRef]
- Nosho, K.; Baba, Y.; Tanaka, N.; Shima, K.; Hayashi, M.; Meyerhardt, J.A.; Giovannucci, E.; Dranoff, G.; Fuchs, C.S.; Ogino, S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: Cohort study and literature review. J. Pathol. 2010, 222, 350–366. [Google Scholar] [CrossRef] [Green Version]
- Kreidieh, M.; Mukherji, D.; Temraz, S.; Shamseddine, A. Expanding the scope of immunotherapy in colorectal cancer: Current clinical approaches and future directions. BioMed Res. Int. 2020, 2020, 9037217. [Google Scholar] [CrossRef] [PubMed]
- Kishore, C.; Bhadra, P. Current advancements and future perspectives of immunotherapy in colorectal cancer research. Eur. J. Pharmacol. 2021, 893, 173819. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, B.; Zhou, Q.; Huang, D. Advances in evidence-based medicine for immunotherapy of non-small cell lung cancer. J. Evid.-Based Med. 2018, 11, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [Green Version]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Narayan, A.; Kole, A.J.; Decker, R.H.; Teysir, J.; Carriero, N.J.; Lee, A.; Nemati, R.; Nath, S.K.; Mane, S.M. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin. Cancer Res. 2018, 24, 1872–1880. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; Hwu, W.-J.; Kefford, R.; Weber, J.S.; Daud, A.; Hamid, O.; Patnaik, A.; Ribas, A.; Robert, C.; Gangadhar, T.C. Evaluation of immune-related response criteria and RECIST v1. 1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. 2016, 34, 1510. [Google Scholar] [CrossRef]
- Persigehl, T.; Lennartz, S.; Schwartz, L.H. iRECIST: How to do it. Cancer Imaging. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98. [Google Scholar] [CrossRef] [Green Version]
- La-Beck, N.M.; Jean, G.W.; Huynh, C.; Alzghari, S.K.; Lowe, D.B. Immune checkpoint inhibitors: New insights and current place in cancer therapy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 255–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- June, C.H.; Warshauer, J.T.; Bluestone, J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017, 23, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Ribas, A. Releasing the brakes on cancer immunotherapy. N. Engl. J. Med. 2015, 373, 1490–1492. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.; Robert, C. Immune checkpoint inhibitors. Immuno-Oncol. 2015, 42, 55–66. [Google Scholar]
- Boussiotis, V.A. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Motzer, R. Fortgeschrittenes Nieren-zellkarzinom: Nivolumab plus Ipilimumab versus Sunitinib. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris Robert, L.; George, B.; Jerome, F.; Joel, G.; Dimitrios, C.A.; Lisa, L.; Kevin, H.; Stefan, K.; Vokes Everett, E.; Caroline, E.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S. Safety and antitumor activity of anti–PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, S.; Lorigan, P. The place of PD-1 inhibitors in melanoma management. Lancet Oncol. 2015, 16, 873–874. [Google Scholar] [CrossRef]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Le, D.T. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015, 373, 1979. [Google Scholar]
- Armand, P.; Nagler, A.; Weller, E.A.; Devine, S.M.; Avigan, D.E.; Chen, Y.-B.; Kaminski, M.S.; Holland, H.K.; Winter, J.N.; Mason, J.R. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: Results of an international phase II trial. J. Clin. Oncol. 2013, 31, 4199. [Google Scholar] [CrossRef] [Green Version]
- Westin, J.R.; Chu, F.; Zhang, M.; Fayad, L.E.; Kwak, L.W.; Fowler, N.; Romaguera, J.; Hagemeister, F.; Fanale, M.; Samaniego, F.; et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: A single group, open-label, phase 2 trial. Lancet Oncol. 2014, 15, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Robert, C. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, S.; Hüllein, J.; Hundemer, M.; Lehners, N.; Jethwa, A.; Capper, D.; Acker, T.; Garvalov, B.K.; Andrulis, M.; Blume, C. Continued response off treatment after BRAF inhibition in refractory hairy cell leukemia. J. Clin.Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, e300-3. [Google Scholar] [CrossRef]
- Tiako Meyo, M.; Jouinot, A.; Giroux-Leprieur, E.; Fabre, E.; Wislez, M.; Alifano, M.; Leroy, K.; Boudou-Rouquette, P.; Tlemsani, C.; Khoudour, N.; et al. Predictive value of soluble PD-1, PD-L1, VEGFA, CD40 ligand and CD44 for nivolumab therapy in advanced non-small cell lung cancer: A case-control study. Cancers 2020, 12, 473. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Lin, J.; Liao, G.; Tian, Y.; Liang, Y.; Li, R.; Liu, M.; Yuan, Y. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e11936. [Google Scholar] [CrossRef] [PubMed]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.A.; Zhuang, T.Z.; Caulfield, S.; Martini, D.J.; Brown, J.T.; Carthon, B.C.; Kucuk, O.; Harris, W.; Bilen, M.A.; Nazha, B. Advances in Knowledge and Management of Immune-Related Adverse Events in Cancer Immunotherapy. Front. Endocrinol. 2022, 354. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Baxi, S.; Yang, A.; Gennarelli, R.L.; Khan, N.; Wang, Z.; Boyce, L.; Korenstein, D. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ 2018, 360, k793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stucci, S.; Palmirotta, R.; Passarelli, A.; Silvestris, E.; Argentiero, A.; Lanotte, L.; Acquafredda, S.; Todisco, A.; Silvestris, F. Immune-related adverse events during anticancer immunotherapy: Pathogenesis and management. Oncol. Lett. 2017, 14, 5671–5680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, B.; Crowe, J.; Graham, R. Rheumatology case report immune-related aortitis associated with ipilimumab. Rheumatologist 2017, 11. [Google Scholar]
- Park, H.; Cho, M.; Do, Y.; Park, J.-K.; Bae, S.-J.; Joo, J.; Ha, K.-T. Autophagy as a Therapeutic Target of Natural Products Enhancing Embryo Implantation. Pharmaceuticals 2022, 15, 53. [Google Scholar] [CrossRef]
- Corb Aron, R.A.; Abid, A.; Vesa, C.M.; Nechifor, A.C.; Behl, T.; Ghitea, T.C.; Munteanu, M.A.; Fratila, O.; Andronie-Cioara, F.L.; Toma, M.M.; Bungau, S. Recognizing the Benefits of Pre-/Probiotics in Metabolic Syndrome and Type 2 Diabetes Mellitus Considering the Influence of Akkermansia muciniphila as a Key Gut Bacterium. Microorganisms 2021, 9, 618. [Google Scholar] [CrossRef]
- Scott, E.S.; Long, G.V.; Guminski, A.; Clifton-Bligh, R.J.; Menzies, A.M.; Tsang, V.H. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur. J. Endocrinol. 2018, 178, 173–180. [Google Scholar] [CrossRef] [PubMed]
- De Velasco, G.; Je, Y.; Bossé, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol. Res. 2017, 5, 312–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.S.; Soni, P.; Chandra, A.B. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front. Pharmacol. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Toma, M.M.; Gitea, D.; Babes, E.E.; Judea Pusta, C.T.; Bumbu, A.G. Current Perspective on the Natural Compounds and Drug Delivery Techniques in Glioblastoma Multiforme. Cancers 2021, 13, 2765. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Islam, M.T.; Harun-Or-Rashid, M.; Islam, M.; Abdullah, S.; Uddin, M.B.; Das, S.; Rahaman, M.S.; Ahmed, M.; et al. Stem Cell Transplantation Therapy and Neurological Disorders: Current Status and Future Perspectives. Biology 2022, 11, 147. [Google Scholar] [CrossRef]
- Karamchandani, D.M.; Chetty, R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: Pathologists’ perspective. J. Clin. Pathol. 2018, 71, 665–671. [Google Scholar] [CrossRef]
- De Martin, E.; Michot, J.-M.; Papouin, B.; Champiat, S.; Mateus, C.; Lambotte, O.; Roche, B.; Antonini, T.M.; Coilly, A.; Laghouati, S.; et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J. Hepatol. 2018, 68, 1181–1190. [Google Scholar] [CrossRef]
- Spain, L.; Diem, S.; Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 2016, 44, 51–60. [Google Scholar] [CrossRef]
- Shahabi, V.; Berman, D.; Chasalow, S.D.; Wang, L.; Tsuchihashi, Z.; Hu, B.; Panting, L.; Jure-Kunkel, M.; Ji, R.-R. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J. Transl. Med. 2013, 11, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, P.D.; Fernandez, A.P.; Velcheti, V.; Tarhini, A.; Funchain, P.; Rini, B.; Khasawneh, M.; Pennell, N.A. Cases from the irAE Tumor Board: A multidisciplinary approach to a patient treated with immune checkpoint blockade who presented with a new rash. Oncologist 2019, 24, 4. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A.T.; Geskin, L. A case of nivolumab-induced bullous pemphigoid: Review of dermatologic toxicity associated with programmed cell death protein-1/programmed death ligand-1 inhibitors and recommendations for diagnosis and management. Oncologist 2018, 23, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjarks, B.J.; Kerkvliet, A.M.; Jassim, A.D.; Bleeker, J.S. Scleroderma-like skin changes induced by checkpoint inhibitor therapy. J. Cutan. Pathol. 2018, 45, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.; Ni, A.; Chaft, J.; Pollina, R.; Kasler, M.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef]
- Marthey, L.; Mateus, C.; Mussini, C.; Nachury, M.; Nancey, S.; Grange, F.; Zallot, C.; Peyrin-Biroulet, L.; Rahier, J.-F.; de Beauregard, M.B.; et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J. Crohn’s Colitis 2016, 10, 395–401. [Google Scholar] [CrossRef]
- Som, A.; Mandaliya, R.; Alsaadi, D.; Farshidpour, M.; Charabaty, A.; Malhotra, N.; Mattar, M.C. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J. Clin. Cases 2019, 7, 405. [Google Scholar] [CrossRef]
- Moein, H.R.; Rutledge, B.; Beydoun, R.; Ehrinpreis, M.N. Ipilimumab and Nivolumab-Induced Colitis in a Patient With Recurrent Metastatic Melanoma. Cureus 2021, 13, e1444. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Richards, C.J.; Boyle, K.; Faust, G. Severe inflammatory ileitis resulting in ileal perforation in association with combination immune checkpoint blockade for metastatic malignant melanoma. Case Rep. 2018, 2018, bcr-2018-224913. [Google Scholar] [CrossRef]
- Reddy, H.G.; Schneider, B.J.; Tai, A.W. Immune checkpoint inhibitor-associated colitis and hepatitis. Clin. Transl. Gastroenterol. 2018, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, P.; Wood, K.; Wassmann, K.; Christenfeld, A.M.; Bhardwaj, N.; Oh, W.K. A whole-blood RNA transcript-based gene signature is associated with the development of CTLA-4 blockade-related diarrhea in patients with advanced melanoma treated with the checkpoint inhibitor tremelimumab. J. Immunother. Cancer 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eigentler, T.K.; Hassel, J.C.; Berking, C.; Aberle, J.; Bachmann, O.; Grünwald, V.; Kähler, K.C.; Loquai, C.; Reinmuth, N.; Steins, M.; et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat. Rev. 2016, 45, 7–18. [Google Scholar] [CrossRef]
- Arriola, E.; Wheater, M.; Karydis, I.; Thomas, G.; Ottensmeier, C. Infliximab for IPILIMUMAB-related colitis. Clin. Cancer Res. 2015, 21, 5642–5643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teufel, A.; Zhan, T.; Härtel, N.; Bornschein, J.; Ebert, M.P.; Schulte, N. Management of immune related adverse events induced by immune checkpoint inhibition. Cancer Lett. 2019, 456, 80–87. [Google Scholar] [CrossRef]
- Johncilla, M.; Misdraji, J.; Pratt, D.S.; Agoston, A.T.; Lauwers, G.Y.; Srivastava, A.; Doyle, L.A. Ipilimumab-associated Hepatitis. Am. J. Surg. Pathol. 2015, 39, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, G.; Bungau, S.; Ceobanu, G.; Ilie, M.; Bacalbasa, N.; Bratu, O.G.; Vesa, C.M.; Gaman, M.-A.; Diaconu, C.C. The non-invasive assessment of hepatic fibrosis. J. Formosan Medical Assoc. 2021, 120, 794–803. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, F.; Afsana Mim, S.; Khan, M.; Islam, M.; Haque, M.; Mitra, S.; Emran, T.B.; Rauf, A. Multifunctional therapeutic approach of nanomedicines against inflammation in cancer and aging. J. Nanomater. 2022, 2022, 4217529. [Google Scholar] [CrossRef]
- Ryder, M.; Callahan, M.; Postow, M.A.; Wolchok, J.; Fagin, J.A. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr.-Relat. Cancer 2014, 21, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Dillard, T.; Yedinak, C.G.; Alumkal, J.; Fleseriu, M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: Serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 2010, 13, 29–38. [Google Scholar] [CrossRef]
- Torino, F.; Barnabei, A.; Paragliola, R.; Marchetti, P.; Salvatori, R.; Corsello, S. Endocrine side-effects of anti-cancer drugs: mAbs and pituitary dysfunction: Clinical evidence and pathogenic hypotheses. Eur. J. Endocrinol. 2013, 169, R153–R164. [Google Scholar] [CrossRef] [PubMed]
- Corsello, S.M.; Barnabei, A.; Marchetti, P.; De Vecchis, L.; Salvatori, R.; Torino, F. Endocrine side effects induced by immune checkpoint inhibitors. J. Clin. Endocrinol. Metab. 2013, 98, 1361–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Rodríguez, E.; Rodríguez-Abreu, D. Immune checkpoint inhibitors: Review and management of endocrine adverse events. Oncologist 2016, 21, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Tabchi, S.; Messier, C.; Blais, N. Immune-mediated respiratory adverse events of checkpoint inhibitors. Curr. Opin. Oncol. 2016, 28, 269–277. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.; Karpinets, T.; Prieto, P.; Vicente, D.; Hoffman, K.; Wei, S.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Tirumani, S.H.; Ramaiya, N.H.; Keraliya, A.; Bailey, N.D.; Ott, P.A.; Hodi, F.S.; Nishino, M. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol. Res. 2015, 3, 1185–1192. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-H.; Zang, X.-Y.; Wang, J.-C.; Huang, S.-S.; Xu, J.; Zhang, P. Diagnosis and management of immune related adverse events (irAEs) in cancer immunotherapy. Biomed. Pharmacother. 2019, 120, 109437. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, F.; Zhu, J.; Chen, Q. Risk of pneumonitis associated with programmed cell death 1 inhibitors in cancer patients: A meta-analysis. Mol. Cancer Ther. 2017, 16, 1588–1595. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, S.; Wang, Y.; Liu, J.; Mao, X.; Xing, H.; Tian, Z.; Tang, K.; Liao, X.; Rao, Q.; et al. Induced CD20 expression on B-cell malignant cells heightened the cytotoxic activity of chimeric antigen receptor engineered T cells. Hum. Gene Ther. 2019, 30, 497–510. [Google Scholar] [CrossRef]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR–T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood J. Am. Soc. Hematol. 2014, 124, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandigursky, S.; Mor, A. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Curr. Rheumatol. Rep. 2018, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Voena, C.; Chiarle, R. Advances in cancer immunology and cancer immunotherapy. Discov. Med. 2016, 21, 125–133. [Google Scholar]
- Fridman, W.H.; Meylan, M.; Petitprez, F.; Sun, C.-M.; Italiano, A.; Sautès-Fridman, C. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol. 2022, 1–17. [Google Scholar] [CrossRef]
- Cassim, S.; Vučetić, M.; Ždralević, M.; Pouyssegur, J. Warburg and beyond: The power of mitochondrial metabolism to collaborate or replace fermentative glycolysis in cancer. Cancers 2021, 12, 1119. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Boon, T.; Cerottini, J.-C.; Van den Eynde, B.; van der Bruggen, P.; Van Pel, A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994, 12, 337–365. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.L.; Bosch, R.J.; Ritz, J.; Hataye, J.M.; Aga, E.; Tressler, R.L.; Mason, S.W.; Hwang, C.K.; Grasela, D.M.; Ray, N.; et al. Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J. Infect. Dis. 2017, 215, 1725–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, S.A.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; Hughes, M.S.; Phan, G.Q.; Citrin, D.E.; Restifo, N.P.; Robbins, P.F.; Wunderlich, J.R.; et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011, 17, 4550–4557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhersecke, L.; Brunet, M.; Guégan, J.-P.; Rey, C.; Bougouin, A.; Cousin, S.; Le Moulec, S.; Besse, B.; Loriot, Y.; Larroquette, M.; et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat. Cancer 2021, 2, 794–802. [Google Scholar] [CrossRef]
- Kudo, M. Immune checkpoint blockade in hepatocellular carcinoma: 2017 update. Liver Cancer 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Cassim, S.; Pouyssegur, J. Tumor microenvironment: A metabolic player that shapes the immune response. Int. J. Mol. Sci. 2019, 21, 157. [Google Scholar] [CrossRef] [Green Version]
- Lassen, U.N.; Makaroff, L.E.; Stenzinger, A.; Italiano, A.; Vassal, G.; Garcia-Foncillas, J.; Avouac, B. Precision oncology: A clinical and patient perspective. Futur. Oncol. 2021, 17, 3995–4009. [Google Scholar] [CrossRef]
- Haanen, J.; Robert, C. Immune checkpoint inhibitors. In Immuno-Oncology; Karger Publishers: Basel, Switzerland, 2015. [Google Scholar]
- Chukwueke, U.N.; Wen, P.Y. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019, 8, CNS28. [Google Scholar] [CrossRef] [Green Version]
- Laparra, A.; Champiat, S.; Michot, J.-M.; Lambotte, O. Management of adverse events associated with cancer immunotherapy. Rev. Prat. 2021, 71, 400–407. [Google Scholar]
- Naing, A.; Hajjar, J.; Gulley, J.L.; Atkins, M.B.; Ciliberto, G.; Meric-Bernstam, F.; Hwu, P. Strategies for improving the management of immune-related adverse events. Cancer 2020, 8, e001754. [Google Scholar] [CrossRef] [PubMed]
- Basse, C.; Swalduz, A.; Levra, M.G.; Girard, N.; Remon, J.; Moro-Sibilot, D. Immunothérapie des cancers bronchiques non à petites cellules métastatiques, de la première ligne à la résistance et sa prise en charge. Bull. Cancer 2020, 107, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Hommes, J.W.; Verheijden, R.J.; Suijkerbuijk, K.P.; Hamann, D. Biomarkers of Checkpoint Inhibitor Induced Immune-Related Adverse Events—A Comprehensive Review. Front. Oncol. 2021, 10, 2916. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.J.; Wolchok, J.D.; Rosenberg, L.M.; Girotra, M. Cancer immunotherapy—Immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 2017, 13, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Yoest, J.M. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: A short review. ImmunoTargets Ther. 2017, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Iwama, S.; Yasuda, Y.; Okada, N.; Tsunekawa, T.; Onoue, T.; Takagi, H.; Hagiwara, D.; Ito, Y.; Morishita, Y.; et al. Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: A prospective study. J. Endocr. Soc. 2018, 2, 241–251. [Google Scholar] [CrossRef]
- Mammen, A.L.; Rajan, A.; Pak, K.; Lehky, T.; Casciola-Rosen, L.; Donahue, R.N.; Lepone, L.M.; Zekeridou, A.; Pittock, S.J.; Hassan, R.; et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann. Rheum. Dis. 2019, 78, 150–152. [Google Scholar] [CrossRef]
- Ali, O.H.; Bomze, D.; Ring, S.S.; Berner, F.; Fässler, M.; Diem, S.; Abdou, M.-T.; Hammers, C.; Emtenani, S.; Braun, A.; et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J. Am. Acad. Dermatol. 2020, 82, 854–861. [Google Scholar]
- Luo, J.; Beattie, J.A.; Fuentes, P.; Rizvi, H.; Egger, J.V.; Kern, J.A.; Leung, D.Y.; Lacouture, M.E.; Kris, M.G.; Gambarin, M.; et al. Beyond Steroids: Immunosuppressants in Steroid-Refractory or Resistant Immune-Related Adverse Events. J. Thorac. Oncol. 2021, 16, 1759–1764. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 2020, 6, 38. [Google Scholar] [CrossRef]
- Myers, G. Immune-related adverse events of immune checkpoint inhibitors: A brief review. Curr. Oncol. 2018, 25, 342–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonomura, Y.; Otsuka, A.; Nakashima, C.; Seidel, J.A.; Kitoh, A.; Dainichi, T.; Nakajima, S.; Sawada, Y.; Matsushita, S.; Aoki, M.; et al. Peripheral blood Th9 cells are a possible pharmacodynamic biomarker of nivolumab treatment efficacy in metastatic melanoma patients. OncoImmunology 2016, 5, e1248327. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Weber, J.; Thompson, J.A.; Hamid, O.; Minor, D.; Amin, A.; Ron, I.; Ridolfi, R.; Assi, H.; Maraveyas, A.; Berman, D.; et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 2009, 15, 5591–5598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, F.; Sykiotis, G.P.; Maillard, M.; Fraga, M.; Ribi, C.; Kuntzer, T.; Michielin, O.; Peters, S.; Coukos, G.; Spertini, F.; et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019, 20, e54–e64. [Google Scholar] [CrossRef] [Green Version]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; Lenihan, D. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Teulings, H.-E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: A systematic review and meta-analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Martini, D.J.; Goyal, S.; Liu, Y.; Evans, S.T.; Olsen, T.A.; Case, K.; Magod, B.L.; Brown, J.T.; Yantorni, L.; Russler, G.A.; et al. Immune-related adverse events as clinical biomarkers in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Oncologist 2021, 26, e1742–e1750. [Google Scholar] [CrossRef]
- Fujii, T.; Colen, R.R.; Bilen, M.A.; Hess, K.R.; Hajjar, J.; Suarez-Almazor, M.E.; Alshawa, A.; Hong, D.S.; Tsimberidou, A.; Janku, F.; et al. Incidence of immune-related adverse events and its association with treatment outcomes: The MD Anderson Cancer Center experience. Investig. New Drugs 2018, 36, 638–646. [Google Scholar] [CrossRef]
- Rizza, L.; Sbardella, E.; Gianfrilli, D.; Lauretta, R.; Tenuta, M.; Del Bene, G.; Longo, F.; Faggiano, A.; Lenzi, A.; Giannetta, E.; et al. Thyroid profile during the alternative Sunitinib dosing 2/1 schedule in metastatic renal cell carcinoma. Endocrine 2020, 67, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Giannetta, E.; La Salvia, A.; Rizza, L.; Muscogiuri, G.; Campione, S.; Pozza, C.; Colao, A.A.L.; Faggiano, A. Are Markers of Systemic Inflammatory Response Useful in the Management of Patients With Neuroendocrine Neoplasms? Front. Endocrinol. 2021, 565. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Guarnotta, V.; De Cicco, F.; Rubino, M.; Faggiano, A.; Colao, A. Immune checkpoint blockade for Merkel cell carcinoma: Actual findings and unanswered questions. J. Cancer Res. Clin. Oncol. 2019, 145, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Hsiehchen, D.; Watters, M.K.; Lu, R.; Xie, Y.; Gerber, D.E. Variation in the assessment of immune-related adverse event occurrence, grade, and timing in patients receiving immune checkpoint inhibitors. JAMA Netw. Open 2019, 2, e1911519. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, A.M.; Al-Oweisi, F.A.; Edwards, S.G.; Al-Nadabi, H.; Al-Fahdi, A.M. Genetic analysis reveals diversity and genetic relationship among Trichoderma isolates from potting media, cultivated soil and uncultivated soil. BMC Microbiol. 2015, 15, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Medications | Molecular Target | Indication | FDA Granted Year [37] |
|---|---|---|---|
| Pembrolizumab | PD-1 | 1. Melanoma | 2014 |
| 2. NSCLC | 2015 | ||
| 3. Hodgkin lymphoma | 2017 | ||
| 4. Urothelial carcinoma | 2017 | ||
| Nivolumab | PD-1 | 1. Melanoma | 2013 |
| 2. NSCLC | 2014 | ||
| 3. Renal cell carcinoma | 2015 | ||
| 4. Hodgkin lymphoma | 2016 | ||
| Durvalumab | PD-L1 | Urothelial carcinoma | 2017 |
| Ipilimumab | CTLA-4 | 1.Melanoma | 2011 |
| 2.Melanoma in combination with nivolumab | 2014 | ||
| Avelumab | PD-L1 | 1.Merkel cell carcinoma | 2017 |
| 2.Urothelial carcinoma | 2017 | ||
| Atezolizumab | PD-L1 | 1.Urothelial carcinoma | 2016 |
| 2.NSCLC | 2016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Behl, T.; Islam, M.R.; Alam, M.N.; Islam, M.M.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Bungau, S.G. Emerging Management Approach for the Adverse Events of Immunotherapy of Cancer. Molecules 2022, 27, 3798. https://doi.org/10.3390/molecules27123798
Rahman MM, Behl T, Islam MR, Alam MN, Islam MM, Albarrati A, Albratty M, Meraya AM, Bungau SG. Emerging Management Approach for the Adverse Events of Immunotherapy of Cancer. Molecules. 2022; 27(12):3798. https://doi.org/10.3390/molecules27123798
Chicago/Turabian StyleRahman, Md. Mominur, Tapan Behl, Md. Rezaul Islam, Md. Noor Alam, Md. Mohaimenul Islam, Ali Albarrati, Mohammed Albratty, Abdulkarim M. Meraya, and Simona Gabriela Bungau. 2022. "Emerging Management Approach for the Adverse Events of Immunotherapy of Cancer" Molecules 27, no. 12: 3798. https://doi.org/10.3390/molecules27123798