Ethanol Determination in Post-Mortem Samples: Correlation between Blood and Vitreous Humor Concentration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Validation
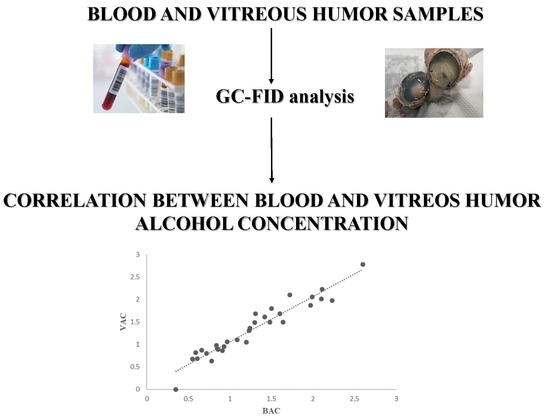
2.2. Ethanol Concentration in Blood and Vitreous Humor
3. Materials and Methods
3.1. Reagents and Solvents
3.2. Samples Collection and Preparation
3.3. Chromatographic Analysis
3.4. Method Validation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ioan, B.G.; Jitaru, V.; Damian, R.; Damian, S.I. Study on the relationship between the concentration of ethanol in the blood, urine and the vitreous humour. Rom. J. Leg. Med. 2015, 23, 211–216. [Google Scholar] [CrossRef]
- Davies, M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. Psychiat Neurosci. 2003, 28, 263–274. [Google Scholar]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019; pp. 1–472. [Google Scholar]
- Vezzoli, S.; Bernini, M.; De Ferrari, F. Ethyl glucuronide in vitreous humor and blood postmortem specimens: Analysis by liquid chromatography electrospray tandem mass spectrometry and interpreting results of neo-formation of ethanol. Ann. Dellt’istituto Super. Sanità 2015, 51, 19–27. [Google Scholar] [CrossRef]
- Holford, N.H.G. Clinical pharmacokinetics of ethanol. Clin. Pharmacokinet. 1987, 13, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Wurst, F.M.; Wiesbeck, G.A.; Metzger, J.W.; Weinmann, W. On sensitivity, specificity, and the influence of various parameters on ethyl glucuronide levels in urine. Results from the WHO/ISBRA study. Alcohol. Clin. Exp. Res. 2004, 28, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Kugelberg, F.C.; Jones, A.W. Interpreting results of ethanol analysis in postmortem specimens: A review of the literature. Forensic Sci. Int. 2007, 165, 10–29. [Google Scholar] [CrossRef]
- Helander, A.; Husa, A.; Jeppsson, J.O. Improved HPLC Method for Carbohydrate-deficient Transferrin in Serum. Clin. Chem. 2003, 49, 1881–1890. [Google Scholar] [CrossRef] [Green Version]
- Skopp, G. Preanalytic aspects in post-mortem toxicology. Forensic Sci. Int. 2004, 142, 75–100. [Google Scholar] [CrossRef]
- Donaldson, A.E.; Lamont, I.L. Biochemistry Changes That Occur after Death: Potential Markers for Determining Post-Mortem Interval. PLoS ONE 2013, 8, e82011. [Google Scholar] [CrossRef] [Green Version]
- Leikin, J.B.; Watson, W.A. Post-mortem toxicology: What the dead can and cannot tell us. J. Toxicol. Clin. Toxicol. 2003, 41, 47–56. [Google Scholar] [CrossRef]
- Butzbach, D.M. The influence of putrefaction and sample storage on post-mortem toxicology results. Forensic Sci. Med. Pathol. 2010, 6, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bevalot, F.; Cartiser, N.; Bottinelli, C.; Fanton, L.; Guitton, J. Vitreous humor analysis for the detection of xenobiotics in forensic toxicology: A review. Forensic Toxicol. 2016, 34, 12–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metushi, I.G.; Fitzgerald, R.L.; McIntyre, I.M. Assessment and Comparison of Vitreous Humor as an Alternative Matrix for Forensic Toxicology Screening by GC–MS. J. Anal. Toxicol. 2016, 40, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Honey, D.; Caylor, C.; Luthi, R.; Kerrigan, S. Comparative alcohol concentrations in blood and vitreous fluid with illustrative case studies. J. Anal. Toxicol. 2005, 29, 365–369. [Google Scholar] [CrossRef] [Green Version]
- Kabir, A.; Furton, K.G.; Tinari, N.; Grossi, L.; Innosa, D.; Macerola, D.; Tartaglia, A.; Di Donato, V.; D’Ovidio, C.; Locatelli, M. Fabric phase sorptive extraction-high performance liquid chromatography-photo diode array detection method for simultaneous monitoring of three inflammatory bowel disease treatment drugs in whole blood, plasma and urine. J. Chromatogr. B 2018, 1084, 53–63. [Google Scholar] [CrossRef]
- Locatelli, M.; Tinari, N.; Grassadoni, A.; Tartaglia, A.; Macerola, D.; Piccolantonio, S.; Sperandio, E.; D’Ovidio, C.; Carradori, S.; Ulusoy, H.I.; et al. FPSE-HPLC-DAD method for the quantification of anticancer drugs in human whole blood, plasma, and urine. J. Chromatogr. B 2018, 1095, 204–213. [Google Scholar] [CrossRef]
- Locatelli, M.; Furton, K.G.; Tartaglia, A.; Sperandio, E.; Ulusoy, H.I.; Kabir, A. An FPSE-HPLC-PDA method for rapid determination of solar UV filters in human whole blood, plasma and urine. J. Chromatogr. B 2019, 1118, 40–50. [Google Scholar] [CrossRef]
- Tartaglia, A.; Kabir, A.; Ulusoy, S.; Sperandio, E.; Piccolantonio, S.; Ulusoy, H.I.; Furton, K.G.; Locatelli, M. FPSE-HPLC-PDA analysis of seven paraben residues in human whole blood, plasma, and urine. J. Chromatogr. B. 2019, 1125, 1–10. [Google Scholar] [CrossRef]
- Tartaglia, A.; Kabir, A.; D’Ambrosio, F.; Ramundo, P.; Ulusoy, S.; Ulusoy, H.I.; Merone, G.M.; Savini, F.; D’Ovidio, C.; De Grazia, U.; et al. Fast off-Line FPSE-HPLC-PDA determination of six NSAIDs in saliva samples. J. Chromatogr. B. 2020, 1144, 1–9. [Google Scholar] [CrossRef]
- Locatelli, M.; Tartaglia, A.; D’Ambrosio, F.; Ramundo, P.; Ulusoy, H.I.; Furton, K.G.; Kabir, A. Biofluid sampler: A new gateway for mail-in-analysis of whole blood samples. J. Chromatogr. B. 2020, 1143, 1–7. [Google Scholar] [CrossRef]
- Winek, C.L.; Esposito, F.M. Comparative study of ethanol levels in blood versus bone marrow, vitreous humor, bile and urine. Forensic Sci. Int. 1981, 17, 27–36. [Google Scholar] [CrossRef]
- Szeremeta, M.; Mironiuk, E.; Janica, M.; Drobuliakova, P.; Lomperta, K.; Szczypek, M.; Janica, A.N. Vitreous humor as an alternative material for the determination of alcohol concentration in human corpses. Arc. Med. Sadowej Kryminol. 2018, 68, 108–118. [Google Scholar] [CrossRef]
- Caplan, Y.H.; Levine, B. Vitreous humor in the evaluation of postmortem blood ethanol concentrations. J. Anal. Toxicol. 1990, 14, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.C.; Lo, D.S. Relationship between postmortem blood and vitreous humor ethanol levels. Am. J. Forensic Med. Pathol. 1993, 14, 303–308. [Google Scholar] [CrossRef] [PubMed]
- CDER e CVM. Bioanalytical Method Validation-Guidance for Industry, Food and Drug Administration. May 2018. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070107.Pdf (accessed on 3 April 2020).
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline (2005) Validation of Analytical Procedures: Text and Methodology Q2(R1), Geneva. 2005. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 3 April 2020).
- GTFI. Guidelines for Laboratories for the Analysis of Substances of Abuse for Toxicological-Forensic and Medico-Legal Purposes, 3rd ed.; GTFI: Pavia, Italy, 2010. [Google Scholar]
Sample Availability: Samples are available from the authors. |



| INTRA-DAY | ||
| Precision (RSD%) | Trueness (Bias%) | |
| 0.01 g/L | 7.9 | 9.2 |
| 0.5 g/L | 6.8 | 7.1 |
| 2 g/L | 5.2 | 5.9 |
| INTER-DAY | ||
| 0.01 g/L | 8.9 | 10.5 |
| 0.5 g/L | 7.6 | 8.1 |
| 2 g/L | 5.6 | 6.1 |
| LLOD | 0.003 g/L | |
| LLOQ | 0.01 g/L | |
| Linearity | 0.01–10 g/L | |
| r2 | 0.9981 ± 0.0025 | |
| Samples | VAC (g/L) | BAC (g/L) | VAC/BAC RATIO |
|---|---|---|---|
| 1 | 1.68 ± 0.09 | 1.31 ± 0.06 | 1.28 ± 0.09 |
| 2 | < LOQ | 0.35 ± 0.03 | - |
| 3 | 0.91 ± 0.04 | 0.85 ± 0.04 | 1.07 ± 0.07 |
| 4 | 2.23 ± 0.19 | 2.11 ± 0.11 | 1.06 ± 0.11 |
| 5 | 1.5 ± 0.09 | 1.64 ± 0.12 | 1.91 ± 0.18 |
| 6 | 0.95 ± 0.06 | 0.93 ± 0.04 | 1.02 ± 0.08 |
| 7 | 1.61 ± 0.08 | 1.42 ± 0.07 | 1.13 ± 0.08 |
| 8 | 1.31 ± 0.09 | 1.23 ± 0.06 | 1.07 ± 0.09 |
| 9 | 1.5 ± 0.11 | 1.48 ± 0.09 | 1.01 ± 0.10 |
| 10 | 2.01 ± 0.18 | 2.10 ± 0.16 | 0.96 ± 0.11 |
| 11 | 1.87 ± 0.12 | 1.97 ± 0.14 | 0.95 ± 0.09 |
| 12 | 1.36 ± 0.09 | 1.24 ± 0.05 | 1.10 ± 0.09 |
| 13 | 0.86 ± 0.04 | 0.91 ± 0.03 | 0.95 ± 0.05 |
| 14 | 1.98 ± 0.16 | 2.23 ± 0.15 | 0.89 ± 0.09 |
| 15 | 0.82 ± 0.06 | 0.59 ± 0.04 | 1.39 ± 0.14 |
| 16 | 1.49 ± 0.07 | 1.30 ± 0.08 | 1.15 ± 0.09 |
| 17 | 2.78 ±0.17 | 2.60 ± 0.17 | 1.07 ± 0.10 |
| 18 | 1.05 ± 0.08 | 1.20 ± 0.08 | 0.88 ± 0.09 |
| 19 | 1.68 ± 0.11 | 1.60 ± 0.11 | 1.05 ± 0.10 |
| 20 | 0.98 ± 0.04 | 0.84 ± 0.03 | 1.17 ± 0.06 |
| 21 | 0.67 ± 0.03 | 0.55 ± 0.02 | 1.22 ± 0.07 |
| 22 | 1.10 ± 0.05 | 1.09 ± 0.05 | 1.01 ± 0.07 |
| 23 | 2.06 ± 0.13 | 1.99 ± 0.13 | 1.04 ± 0.09 |
| 24 | 0.80 ± 0.07 | 0.72 ± 0.03 | 1.11 ± 0.11 |
| 25 | 1.06 ± 0.05 | 0.97 ± 0.09 | 1.09 ± 0.11 |
| 26 | 0.87 ± 0.04 | 0.66 ± 0.06 | 1.32 ± 0.13 |
| 27 | 2.10 ± 0.16 | 1.72 ± 0.09 | 1.22 ± 0.11 |
| 28 | 1.80 ± 0.11 | 1.50 ± 0.07 | 1.20 ± 0.09 |
| 29 | 0.68 ± 0.05 | 0.61 ± 0.02 | 1.11 ± 0.09 |
| 30 | 0.89 ± 0.06 | 0.86 ± 0.04 | 1.03 ± 0.08 |
| 31 | 0.63 ± 0.06 | 0.78 ± 0.03 | 0.81 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savini, F.; Tartaglia, A.; Coccia, L.; Palestini, D.; D’Ovidio, C.; de Grazia, U.; Merone, G.M.; Bassotti, E.; Locatelli, M. Ethanol Determination in Post-Mortem Samples: Correlation between Blood and Vitreous Humor Concentration. Molecules 2020, 25, 2724. https://doi.org/10.3390/molecules25122724
Savini F, Tartaglia A, Coccia L, Palestini D, D’Ovidio C, de Grazia U, Merone GM, Bassotti E, Locatelli M. Ethanol Determination in Post-Mortem Samples: Correlation between Blood and Vitreous Humor Concentration. Molecules. 2020; 25(12):2724. https://doi.org/10.3390/molecules25122724
Chicago/Turabian StyleSavini, Fabio, Angela Tartaglia, Ludovica Coccia, Danilo Palestini, Cristian D’Ovidio, Ugo de Grazia, Giuseppe Maria Merone, Elisa Bassotti, and Marcello Locatelli. 2020. "Ethanol Determination in Post-Mortem Samples: Correlation between Blood and Vitreous Humor Concentration" Molecules 25, no. 12: 2724. https://doi.org/10.3390/molecules25122724