3. Materials and Methods
All starting materials purchased from Sigma-Aldrich and Fluka chemical companies were used without further purification.
1H,
13C, and
19F NMR spectra were registered with Bruker Avance 400 and Agilent 400 MR spectrometers (400, 100.6, and 376.4 MHz, respectively) in CDCl
3 at 298K using residual peaks of the solvent as standards. MALDI-TOF mass spectra were registered with Bruker Autoflex II mass spectrometer in positive mode using dithranol as matrix and poly(ethylene)glycols as internal standards. Preparative column chromatography was performed using silica gel from Merck Co (40/60). The syntheses under the same conditions were conducted using Radleys Carousel 12 Plus reaction station. Pd(dba)
2 was obtained via a procedure described in [
31].
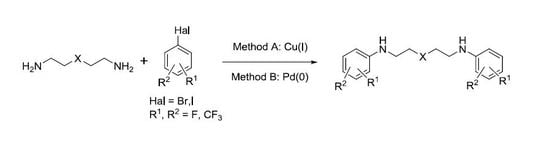
General method (A) for Cu(I)-catalyzed N,N′-diarylation of oxadiamines. The reaction vessel was flushed with dry argon, equipped with a magnetic stirrer and reflux condenser, and charged with CuI and the ligand (2-isobutyrylcyclohexanone or rac-BINOL); 1.25 mmol of the appropriate aryl halide and dry DMF (1 mL) were added followed by the oxadiamine (0.5 mmol). The reaction mixture was stirred for several minutes, then Cs2CO3 (1.25 mmol) was added and the reaction mixture was stirred at 140 °C for 24 h to ensure the full completion of the process. Next, the reaction mixture was cooled to ambient temperature, a small amount of the solution was taken for 1H NMR investigation of its composition, dichloromethane (5–10 mL) was added, the residue filtered off washed with dichloromethane (5–10 mL), and the combined organic fractions were evaporated in vacuo and chromatographed on silica gel using a sequence of eluents: CH2Cl2, CH2Cl2-MeOH (200:1, 100:1, 50:1, 20:1, 10:1).
General method (B) for Pd(0)-catalyzed N,N′-diarylation of oxadiamines. The reaction vessel was flushed with dry argon, equipped with a magnetic stirrer and reflux condenser, and charged with Pd(dba)2 and BINAP ligand, corresponding to aryl halide (1–1.25 mmol) and 5 mL absolute dioxane. After stirring the mixture for several minutes, the appropriate oxadiamine (0.5 mmol) and tBuONa (1.5 mmol) were added. The reaction mixture was refluxed for 8 h, and its work up was essentially the same as described for Cu(I)-catalyzed arylation.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(4-fluoroaniline) (4). Obtained according to method B from trioxadiamine 1 (0.5 mmol, 110 mg), 4-bromofluorobenzene (1.25 mmol, 218 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2–MeOH 100:1. Yield 147 mg (72%). 1H-NMR (400 MHz, CDCl3) δ 1.86 (quintet, 4H, 3J = 6.1 Hz, CH2CH2N), 3.17 (t, 4H, 3J = 6.4 Hz, CH2N), 3.56–3.61 (m, 8H, OCH2), 3.64–3.67 (m, 4H, OCH2), 4.34 (br. s, 2H, NH), 6.55 (dd, 4H, 3JHH = 8.8 Hz, 4JHF= 4.3 Hz, H2, H2′ (Ph)), 6.84–6.85 (m, 4H, H3, H3′ (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.6 (2C, CH2CH2N), 42.1 (2C, CH2N), 69.4 (2C, OCH2), 70.1 (2C, OCH2), 70.4 (2C, OCH2), 113.6 (d, 4C, 3JCF = 6.6 Hz, C2, C2′(Ph)), 115.1 (d, 4C, 2JCF = 22.1 Hz, C3, C3′(Ph)), 144.5 (2C, C1(Ph)), 155.1 (d, 2C, 2JCF = 235.1 Hz, C4 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –127,30 br. s. MS (MALDI-TOF+): Calculated for C22H31F2N2O3 [M + H] 409.2303, found 409.2267.
N,N′-(((oxybis(ethan-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(3-fluoroaniline) (5). Obtained according to method A from trioxadiamine 1 (0.5 mmol, 110 mg), 3-fluoroiodobenzene (1.25 mmol, 278 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2–MeOH 100:1. Yield 167 mg (82%). 1H-NMR (400 MHz, CDCl3) δ 1.88 (quintet, 4H, 3J = 6.1 Hz, CH2CH2N), 3.21 (q, 4H, 3J = 6.4 Hz, CH2N), 3.59–3.64 (m, 8H, OCH2), 3.68–3.70 (m, 4H, OCH2), 4.25 (br. s, 2H, NH), 6.32–6.37 (m, 4H, H4, H6 (Ph)), 6.28 (dt, 2H, 3JHF = 11.8 Hz, 4JHH = 2.3 Hz, H2 (Ph)); 7.06 (td, (2H, 3JHH = 8.2 Hz, 4JHF = 6.8 Hz, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.4 (2 C, CH2CH2N), 41.5 (2C, CH2N), 69.4 (2C, OCH2), 69.8 (2C, OCH2), 70.2 (2 C, OCH2), 98.8 (d, 2C, 2JCF = 25.2 Hz, C2(Ph)), 102.8 (d, 2C, 2JCF = 21.6 Hz, C4(Ph)), 108.8 (2C, C6(Ph)), 129.7 (d, 2C, 3JCF = 10.3 Hz, C5(Ph)), 150.0 (d, 2C, 3JCF = 11.1 Hz, C1(Ph)), 163.7 (d, 2C, 1JCF = 241.6 Hz, C3(Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –113.05 (ddd, 3JHF = 11.8 Hz, 3JHF = 8.9 Hz, 4JHF = 6.8 Hz). MS (MALDI-TOF+): Calculated for C22H31F2N2O3 [M + H] 409.230, found 409.244.
N,N′-(((oxybis(ethan-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(2-fluoroaniline) (6). Obtained according to method B from trioxadiamine 1 (0.5 mmol, 110 mg), 2-bromofluorobenzene (1.25 mmol, 218 mg) in the presence of Pd(dba)2 (11 mg) and BINAP (14 mg). Eluent CH2Cl2–MeOH 200:1. Yield 200 mg (98%). 1H-NMR (400 MHz, CDCl3) δ 1.91 (quintet, 4H, 3J = 6.1 Hz, CH2CH2N), 3.25 (t, 4H, 3J = 6.5 Hz, CH2N), 3.59–3.62 (m, 8H, OCH2), 3.67–3.69 (m, 4H, OCH2), 4.21 (br. s, 2H, NH), 6.58 (ddd, 2H, 3JHH = 7.8 Hz, 4JHF = 5.3 Hz, 4JHH = 1.1 Hz, H4 (Ph)), 6.67–6.71 (m, 2H, H6(Ph)), 6.95 (ddd, 2H, 3JHH = 8.0 Hz, 3JHF = 11.9 Hz, 4JHH = 1.1 Hz, H3(Ph)), 6.98 (t, 2H, 3J = 8.0 Hz, H5(Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.7 (2C, CH2CH2N), 41.0 (2C, CH2N), 69.3 (2C, OCH2), 70.0 (2C, OCH2), 70.3 (2C, OCH2), 111.5 (2C, C6 (Ph)), 113.8 (d, 2C, 2JCF = 18.6 Hz, C3 (Ph)), 115.8 (d, 2C, 3JCF = 6.8 Hz, C4 (Ph)), 124.2 (2C, C5 (Ph)), 136.5 (d, 2C, 3JCF = 11.6 Hz, C1 (Ph)), 151.2 (d, 2C, 2JCF = 237.9 Hz, C2 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –136.80 (ddd, 3JHF= 11.9 Hz, 4JHF= 6.7 Hz, 4JHF= 5.3 Hz). MS (MALDI-TOF+): Calculated for C22H31F2N2O3 [M + H] 409.2303, found 409.2345.
N,N′-((ethane-1,2-diyl(oxy))bis(ethane-2,1-diyl))bis(4-fluoroaniline) (7). Obtained according to method B from dioxadiamine 2 (0.5 mmol, 74 mg), 4-bromofluorobenzene (1.25 mmol, 218 mg) in the presence of Pd(dba)2 (5.7 mg) and BINAP (7.8 mg). Eluent CH2Cl2–MeOH 200:1. Yield 123 mg (73%). 1H-NMR (400 MHz, CDCl3) δ 3.26 (t, 4H, 3J = 5.2 Hz, CH2NH), 3.66 (s, 4H, OCH2CH2O), 3.72 (t, 4H, 3J = 5.1 Hz, OCH2CH2N), 3,98 (br.s, 2H, NH), 6.63 (dd, 4H, 3JHH = 8.8 Hz, 4JHF = 4.4 Hz, H2, H2′ (Ph)), 6.85–6.90 (m, 4H, H3, H3′ (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 43.8 (2C, CH2N), 69.1 (2C, OCH2CH2N), 69.8 (2C, OCH2CH2O), 113.6 (d, 4C, 3JCF= 7.0 Hz, C2, C2′ (Ph)), 115.2 (d, 4C, 2JCF = 22.3 Hz, C3, C3′ (Ph)), 144.1 (2 C, C1 (Ph)), 155.5 (d, 2 C, 1JCF = 235 Hz, C4 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –136.80 (tt, 3JHF= 8.7 Hz, 4JHF = 4.4 Hz). MS (MALDI-TOF+): Calculated for C18H23F2N2O2 [M + H] 337.1728, found 337.1702.
4-Fluoro-N-(4-fluorophenyl)-N-(2-(2-(2-((4-fluorophenyl)amino)ethoxy)ethoxy)ethyl)aniline (7a). Obtained as the second product in the synthesis of compound 7 using method B. Eluent CH2Cl2. Yield 24 mg (11%). 1H-NMR (400 MHz, CDCl3) δ 3.25 (t, 2H, 3J = 5.2 Hz, CH2NHAr), 3.59 (s, 4H, OCH2CH2O), 3.64 (t, 2H, 3J = 5.2 Hz, OCH2CH2NHAr), 3.67 (t, 2H, 3J = 6.1 Hz, OCH2CH2NAr2), 3.83 (t, 2H, 3J = 6.1 Hz, CH2NAr2), 6.62 (dd, 2H, 3JHH = 8.8 Hz, 4JHF = 4.3 Hz, H (Ph)), 6.89 (t, 2H, 3JHH = 8.7 Hz 2H, H (Ph)), 6.92-6.95 (m, 8H, H (Ph)), NH proton was not unambiguously assigned. 13C-NMR (100.6 MHz, CDCl3) δ 45.8 (1C, CH2NHAr), 52.1 (1C, CH2NAr2), 68.2 (1C, OCH2), 68.5 (1C, OCH2), 68.5 (1C, OCH2), 70.3 (1C, OCH2), 70.6 (1C, OCH2), 115.8 (d, 6C, 2JCF = 22.1 Hz, CH (Ph)), 116.4 (br. s, 2C, CH (Ph)), 122.3 (d, 4C, 3JCF = 7.4 Hz, CH (Ph)), 144.3 (2C, CN (Ph)), 157.9 (d, 2C, 1JCF = 240.9 Hz, CF (Ph)), two quaternary carbon atoms were not unambiguously assigned. MS (MALDI-TOF+): Calculated for C24H26F3N2O2 [M + H] 431.195, found 431.184.
N,N′-((ethane-1,2-diyl(oxy))bis(ethane-2,1-diyl))bis(3-fluoroaniline) (8). Obtained according to method A from dioxadiamine 2 (0.5 mmol, 74 mg), 3-fluoroiodobenzene (1.25 mmol, 278 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2–MeOH 200:1. Yield 118 mg (70%). 1H-NMR (400 MHz, CDCl3) δ 3.26 (t, 4H, 3J = 5.2 Hz, CH2NH), 3.66 (s, 4H, OCH2CH2O), 3.72 (t, 4H, 3J = 5.1 Hz, OCH2CH2N), 4.01 (br. s, 2H, NH), 6.30 (dt, 2H, 3JHF = 11.5 Hz, 4JHH = 2.1 Hz, H2 (Ph)), 6.36–6.41 (m, 4H, H4, H6 (Ph)), 7.08 (td, 2H, 3JHH = 8.0 Hz, 4JHF = 6.9 Hz, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 43.1 (2C, CH2N), 69.0 (2C, OCH2CH2N), 69.8 (2C, OCH2CH2O), 99.3 (d, 2C, 2JCF = 25.4 Hz, C2 (Ph)), 103.6 (d, 2C, 2JCF = 21.4 Hz, C4 (Ph)), 108.6 (2C, C6 (Ph)), 129.8 (d, 2C, 3JCF = 10.1 Hz, C5 (Ph)), 149.4 (d, 2C, 3JCF = 10.9 Hz, C1 (Ph)), 163.7 (d, 2C, 1JCF = 242.7 Hz, C3 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –136.80 (ddd, 3JHF = 11.5 Hz, 3JHF = 6.9 Hz, 4JHF = 2.1 Hz). MS (MALDI-TOF+): Calculated for C18H21F2N2O2 [M – H2 + H] 335.1571, found 335.1546.
N,N′-((ethane-1,2-diyl(oxy))bis(ethane-2,1-diyl))bis(2-fluoroaniline) (9). Obtained according to method B from dioxadiamine 2 (0.5 mmol, 74 mg), 2-bromofluorobenzene (1.25 mmol, 218 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2–MeOH 200:1. Yield 105 mg (63%). 1H-NMR (400 MHz, CDCl3) δ 3.34 (t, H, 3J = 5.2 Hz, CH2NH,), 3.67(s, 4H, OCH2CH2O), 3.73 (t, 4H, 3J = 5.2 Hz, OCH2CH2N), 4.30 (br. s, 2H, NH), 6.63 (td, 2H, 3JHH = 7.2 Hz, 4JHF = 5.7 Hz, H4 (Ph)), 6.70–6.74 (m, 2H, H6 (Ph)), 6.93–7.00 (m, 4H, H3, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 42.9 (2C, CH2N), 69.1 (2C, OCH2CH2N), 70.0 (2C, OCH2CH2O), 112.1 (2C, C6 (Ph)), 114.1 д (2C, 2JCF = 18.6 Hz, C3 (Ph)), 116.6 d (2C, 3JCF = 6.4 Hz, C4 (Ph)), 124.1 (2C, C5 (Ph)), 136.1 д (2C, 2JCF = 11.2 Hz, C1 (Ph)), 151.4 д (2 C, 2JCF = 239.2 Hz, C2 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –136.10 br. s. MS (MALDI-TOF+): Calculated for C18H21F2N2O2 [M – H2+ H] 335.1571, found 335.1552.
N-(2-(2-(2-aminoethoxy)ethoxy)ethyl)-2-fluoroaniline (9a). Obtained as the second product in the synthesis of compound 9 using method A. Eluent CH2Cl2–MeOH 50:1. Yield 19 mg (16%). 1H-NMR (400 MHz, CDCl3) δ 3.32–3.35 (m, 2H, CH2N), 3.50 (q, 2H, 3J = 5.0 Hz, CH2N), 3.58 (t, 4H, 3J = 4.8 Hz, OCH2), 3.64–3.66 (m, 2H, OCH2), 3.73 (t, 2H, 3J = 5.2 Hz, OCH2), 6.14 (br. s, 1H, NH), 6.62–6.69 (m, 1H, H4(Ph)), 6.73 (dd, 1H, 3JHF = 8.2 Hz, 3JHH = 8.2 Hz, H6(Ph)), 6.94–7.00 (m, 1H, H3(Ph)), 7.00 (dd, 1H, 3JHFobs = 8.0 Hz, H5(Ph)), NH2 protons were not unambiguously assigned. MS (MALDI-TOF+): Calculated for C12H20FN2O2 [M + H] 243.1508, found 243.1466.
N,N′-((butane-1,4-diylbis(oxy))bis(propane-3,1-diyl))bis(4-fluoroaniline) (10). Obtained according to method B from dioxadiamine 3 (0.5 mmol, 102 mg), 4-bromofluorobenzene (1.25 mmol, 218 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2–MeOH 200:1. Yield 118 mg (60%). 1H-NMR (400 MHz, CDCl3) δ 1.65–1.67 (m, 4H, OCH2CH2CH2CH2O), 1.87 (quintet, 4H, 3J = 6.0 Hz, OCH2CH2CH2N), 3.17 (t, 4H, 3J = 6.5 Hz, CH2N), 3.43–3.46 (m, 4H, OCH2CH2CH2CH2O), 3.53 (t, 4H, 3J = 5.8 Hz, OCH2CH2CH2N), 4.09 (br. s, 2H, NH), 6.54 (dd, 4H, 3JHH = 8.8 Hz, 4JHF = 4.4 Hz, H2, H2′ (Ph)), 7.85–7.89 (m, 4H, H3, H3′ (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 26.2 (2C, CH2CH2CH2CH2), 28.8 (2C, OCH2CH2CH2NH), 42.6 (2C, CH2N), 69.2 (2C, OCH2), 70.4 (2C, OCH2), 113.3 (d, 4C, 3JCF = 7.2 Hz, C2, C2′ (Ph)), 115.2 (d, 4C, 2JCF = 22.3 Hz, C3, C3′ (Ph)), 144.2 (2C, C1 (Ph)), 155.4 (d, 2C, 1JCF = 234.4 Hz, C4 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –126.97 br. s. MS (MALDI-TOF+): Calculated for C22H31F2N2O2 [M + H] 393.2354, found 393.2386.
N,N′-((butane-1,4-diylbis(oxy))bis(propane-3,1-diyl))bis(3-fluoroaniline) (11). Obtained according to method A from dioxadiamine 3 (0.5 mmol, 102 mg), 3-fluoroiodobenzene (1.25 mmol, 278 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2–MeOH 200:1. Yield 151 mg (77%). 1H-NMR (400 MHz, CDCl3) δ 1.69–1.71 (m, 4H, OCH2CH2CH2CH2O), 1.88 (quintet, 4H, 3J = 6.1 Hz, OCH2CH2CH2N), 3.25 (t, 4H, 3J = 6.3 Hz, CH2N), 3.46–3.48 (m, 4H, OCH2), 3.55 (t, 4H, 3J = 5.7 Hz, OCH2), 4.20 (br. s, 2H, NH), 6.32 (dt, 2H, 3JHF = 11.8 Hz, 4JHH = 2.2 Hz, H2 (Ph)), 6.36–6.40 (m, 4 H, H4, H6 (Ph)), 7.10 (td, 2H, 3JHH = 7.6 Hz, 4JHF = 7.6 Hz, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 26.2 (2C, CH2CH2CH2CH2), 28.8 (2C, OCH2CH2CH2NH), 41.6 (2C, CH2N), 69.0 (2C, OCH2), 70.5 (2C, OCH2), 98.7 (d, 2C, 2JCF = 25.3 Hz, C2 (Ph)), 102.8 (d, 2C, 2JCF = 21.6 Hz, C4 (Ph)), 108.2 (2C, C6 (Ph)), 129.8 (d, 2C, 3JCF = 10.1 Hz, C5 (Ph)), 150.0 (d, 2C, 3JCF = 7.7 Hz, C1 (Ph)), 163.8 (d, 2C, 1JCF = 241.8 Hz, C3 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –113.10 (ddd, 3JHF= 11.8 Hz, 3JHF = 8.7 Hz, 4JHF = 7.6 Hz). MS (MALDI-TOF+): Calculated for C22H31F2N2O2 [M + H] 393.2354, found 393.2329.
N,N′-((butane-1,4-diylbis(oxy))bis(propane-3,1-diyl))bis(2-fluoroaniline) (12). Obtained according to method B from dioxadiamine 3 (0.5 mmol, 102 mg), 2-bromofluorobenzene (1.25 mmol, 218 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2–MeOH 200:1. Yield 96 mg (49%). 1H-NMR (400 MHz, CDCl3) δ 1.67–1.70 (m, 4H, OCH2CH2CH2CH2O), 1.91 (quintet, 4H, 3J = 6.1 Hz, OCH2CH2CH2N), 3.26 (t, 4H, 3J = 6.2 Hz, CH2N), 3.44–3.47 (m, 4H, OCH2), 3.55 (t, 4H, 3J = 5.8 Hz, OCH2), 4.32 (br. s, 2H, NH), 6.58 (tdd, 2H, 3JHH = 7.6 Hz, 4JHF = 4.9 Hz, 4JHH = 1.5 Hz, H4 (Ph)), 6.67–6.72 (m, 2H, H6 (Ph)), 6.95 (ddd, 2H, 3JHF = 11.9 Hz, 3JHH = 8.2 Hz, 4JHH = 1.4 Hz, H3 (Ph)), 6.98 (t, 2H, 3J = 8.0 Hz, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 26.1 (2C, CH2CH2CH2CH2), 28.9 (2C, OCH2CH2CH2NH), 41.3 (2C, CH2N), 68.9 (2C, OCH2), 70.5 (2C, OCH2), 111.4 (2C, C6 (Ph)), 113.8 (d, 2C, 2JCF = 18.1 Hz, C3 (Ph)), 115.7 (d, 2C, 3JCF = 6.6 Hz, C4 (Ph)), 124.1 (2C, C5 (Ph)), 136.7 (d, 2C, 3JCF = 11.6 Hz, C1 (Ph)), 151.1 (d, 2C, 2JCF = 238.5 Hz, C2 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –136.88 (ddd, 3JHF = 11.9 Hz, 3JHF = 6.5 Hz, 4JHF = 4.9 Hz). MS (MALDI-TOF+): Calculated for C22H31F2N2O2 [M + H] 393.2354, found 393.2332.
N-(3-(4-(3-aminopropoxy)butoxy)propyl)-2-fluoroaniline (12a). Obtained as the second product in the synthesis of compound 12 using method A. Eluent CH2Cl2–MeOH 50:1. Yield 9126 mg (8%). 1H-NMR (400 MHz, CDCl3) δ 1.63–1.68 (m, 4H, OCH2CH2CH2CH2O), 1.78 (quintet, 2H, 3J = 6.0 Hz, OCH2CH2CH2NH2), 1.91 (quintet, 2H, 3J = 6.0 Hz, OCH2CH2CH2NH), 3.25 (t, 2H, 3J = 5.9 Hz, CH2N), 3.38–3.46 (m, 6H, CH2N, OCH2), 3.51 (t, 2H, 3J = 5.7 Hz, OCH2), 3.56 (t, 2H, 3J = 5.7 Hz, OCH2), 6.22 (br. s, 1H, NH), 6.56–6.62 (m, 1H, H4 (Ph)), 6.69 (dd, 1H, 3JHH = 8.3 Hz, 4JHF = 8.3 Hz, H6 (Ph)), 6.94 (dd, 1H, 3JHF = 12.0 Hz, 3JHH = 8.1 Hz, H3 (Ph)), 6.98 (t, 1H, 3Jobs = 7.7 Hz, H5 (Ph)). NH2 protons were not unambiguously assigned. MS (MALDI-TOF+): Calculated for C16H28FN2O2 [M + H] 299.2135, found 299.2114.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(2,4-difluoroaniline) (13). Obtained according to method A from trioxadiamine 1 (0.5 mmol, 110 mg), 2,4-difluoro-1-iodobenzene (1.25 mmol, 300 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2–MeOH 200:1. Yield 45 mg (20%). 1H-NMR (400 MHz, CDCl3) δ 1.89 (quintet, 4H, 3J = 6.0 Hz, OCH2CH2CH2NH), 3.21 (t, 4H, 3J = 6.3 Hz, CH2N), 3.58–3.60 (m, 8H, OCH2), 3.65–3.67 (m, 4H, OCH2), 6.63 (td, 2H, 3JHF = 9.0 Hz, 3JHF = 5.8 Hz, H3 (Ph)), 6.70–6.77 (m, 4H, H5, H6 (Ph)), NH were not unambiguously assigned. 13C-NMR (100.6 MHz, CDCl3) δ 28.5 (2C, OCH2CH2CH2NH), 42.2 (2C, CH2N), 69.3 (2C, OCH2), 70.0 (2C, OCH2), 70.2 (2C, OCH2), 103.0 (t, 2C, 2JCFobs = 24.0 Hz, C3 (Ph)), 110.1 (d, 2C, 2JCF = 22.7 Hz, C5 (Ph)), 112.3 (br. s, 2C, C6 (Ph)), quaternary carbon atoms were not unambiguously assigned due to low intensity of their multiplets. 19F-NMR (376.4 MHz, CDCl3) δ –107.02 (qd, 2F, 3JHF = 4JFF = 9.0 Hz, 4JHF = 6.3 Hz 4-F), (−115.35)–(−115.43) (m, 2F, 2-F). MS (MALDI-TOF+): Calculated for C22H29F4N2O3 [M + H] 445.2114, found 445.2139.
N-(3-(2-(2-(3-aminopropoxy)ethoxy)ethoxy)propyl)-2,4-difluoroaniline (13a). Obtained as the second product in the synthesis of compound 13 using method A. Eluent CH2Cl2–MeOH 50:1. Yield 32 mg (19%). 1H-NMR (400 MHz, CDCl3) δ 1.76 (quintet, 2H, 3J = 5.7 Hz, OCH2CH2CH2NH2), 1.89 (quintet, 2H, 3J = 5.7 Hz, OCH2CH2CH2NHAr), 3.21 (t, 2H, 3J = 5.1 Hz, CH2NH), 3.39 (q, 2H, 3J = 5.4 Hz, CH2N), 3.50–3.67 (m, 12 H, OCH2), 6.55–6.63 (m, 1H, H3(Ph)), 6.69–6.76 (m, 2H, H5, H6 (Ph)), NH and NH2 protons were not unambiguously assigned. MS (MALDI-TOF+): Calculated for C16H27F2N2O3 [M + H] 333.1990, found 333.1964.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(4-(trifluoromethyl)aniline) (14). Obtained according to method A from trioxadiamine 1 (0.5 mmol, 110 mg), 1-iodo-4-(trifluoromethyl)benzene (1.25 mmol, 340 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2–MeOH 100:1. Yield 64 mg (25%). 1H-NMR (400 MHz, CDCl3) δ 1.87 (quintet, 4H, 3J = 6.0 Hz, CH2CH2N), 3.24 (t, 4H, 3J = 6.4 Hz, CH2N), 3.57–3.62 (m, 8H, OCH2), 3.66–3.69 (m, 4H, OCH2), 4.60 (br. s, 2H, NH), 6.56 (d, 4H, 3Jobs = 8.6 Hz, H2, H2′ (Ph)), 7.36 (d, 4H, 3Jobs = 8.6 Hz, H3, H3′ (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.7 (2C, CH2CH2N), 41.5 (2C, CH2N), 69.7 (2C, OCH2), 70.2 (2C, OCH2), 70.6 (2C, OCH2), 113.6 (4C, C2, C2′ (Ph)), 118.2 (q, 2C, 2JCF= 32.0 Hz, C4 (Ph)), 122.4 (q, 2C, 1JCF = 270.6 Hz, CF3), 126.7 (4C, C3, C3′ (Ph)), 150.9 (2C, C1 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –61.13 (6F, CF3). MS (MALDI-TOF+): Calculated for C24H30F5N2O3 [M–F] 489.2177, found 489.2198.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(3-(trifluoromethyl)aniline) (15). Obtained according to method A from trioxadiamine 1 (0.5 mmol, 110 mg), 1-iodo-3-(trifluoromethyl)benzene (1.25 mmol, 340 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2–MeOH 200:1. Yield 53 mg (21%). 1H-NMR (400 MHz, CDCl3) δ 1.87 (quintet, 4H, 3J = 5.9 Hz, CH2CH2N), 3.23 (t, 4H, 3J = 6.3 Hz, CH2N), 3.58–3.61 (m, 8H, OCH2), 3.66–3.69 (m, 4H, OCH2), 4.22 (br. s, 2H, NH), 6.71 (d, 2H, 3J = 6.6 Hz, H6 (Ph)), 6.77 (s, 2H, H2 (Ph)), 6.88 (d, 2H, 3J = 8.0 Hz, H4 (Ph)), 7.21 (t, 2H, 3Jobs = 8.0 Hz, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.7 (2C, CH2CH2NH), 41.8 (2C, CH2N), 69.8 (2C, OCH2), 70.2 (2C, OCH2), 70.5 (2C, OCH2), 108.7 (2C, C2 (Ph)), 113.2 (2C, C4 (Ph)), 115.6 (2C, C6 (Ph)), 124.4 (q, 2C, 1JCF = 272.2 Hz, CF3), 129.5 (2C, C5 (Ph)), 131.3 (q, 2C, 2JCF = 32.4 Hz, C3 (Ph)), 148.6 (2C, C1 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –62.85 (6F, CF3). MS (MALDI-TOF+): Calculated for C24H31F6N2O3 [M + H] 509.224, found 509.233.
N,N′-((ethane-1,2-diyl(oxy))bis(ethane-2,1-diyl))bis(4-(trifluoromethyl)aniline) (16). Obtained according to method A from dioxadiamine 2 (0.5 mmol, 74 mg), 1-iodo-4-(trifluoromethyl)benzene (1.25 mmol, 340 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2–MeOH 200:1. Yield 31 mg (14%). 1H-NMR (400 MHz, CDCl3) δ 3.32 (t, 4H, 3J = 5.3 Hz, CH2NH), 3.66 (s, 4H, OCH2CH2O), 3.71 (t, 4 H, 3J = 5.3 Hz, OCH2), 3.93 (br. s, 2H, NH), 6.61 (d, 4H, 3J = 8.5 Hz, H2, H2′ (Ph)), 7.37 (d, 4H, 3J = 8.5 Hz, H3, H3′ (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 43.1 (2C, CH2N), 69.2 (2C, OCH2), 70.2 (2C, OCH2), 112.2 (4C, C2, C2′ (Ph)), 119.2 (q, 2C, 2JCF = 32.0 Hz, C4 (Ph)), 122.2 (q, 2C, 1JCF = 279.1 Hz, CF3 (Ph)), 126.6 (br. s, 4C, C3, C3′ (Ph)), 150.3 (2C, C1 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –61.19 (6F, CF3). MS (MALDI-TOF+): Calculated for C20H22F5N2O2 [M – F] 417.1601, found 417.1581.
N,N′-((ethane-1,2-diyl(oxy))bis(ethane-2,1-diyl))bis(3-(trifluoromethyl)aniline) (17). Obtained according to method A from dioxadiamine 2 (0.5 mmol, 74 mg), 1-iodo-3-(trifluoromethyl)benzene (1.25 mmol, 340 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2–MeOH 200:1. Yield 214 mg (98%). 1H-NMR (400 MHz, CDCl3) δ 3.30 (t, 4H, 3J = 5.2 Hz, CH2N), 3.66 (s, 4H, OCH2CH2O), 3.71 (t, 4H, 3J = 5.2 Hz, CH2O), 4.22 (br. s, 2H, NH), 6.73 (dd, 2H, 3J = 8.0 Hz, 4J = 2.0 Hz, H6(Ph)), 6.81 (s, 2H, H2(Ph)), 6.93 (d, 2H, 3J = 7.6 Hz, H4(Ph)), 7.22 (t, 2H, 3Jobs = 7.9 Hz, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 43.2 (2C, CH2N), 69.3 (2C, OCH2), 70.2 (2C, OCH2), 108.9 (2C, C2(Ph)), 113.6 (2C, C4(Ph)), 116.0 (2C, C6(Ph)), 124.3 (q, 2C, 1JCF = 272.0 Hz, CF3), 129.5 (2C, C5(Ph)), 131.4 (q, 2C, 2JCF = 31.9 Hz, C3(Ph)), 148.3 (2C, C1 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –62.87 (6F, CF3). MS (MALDI-TOF+): Calculated for C20H22F6N2O2 [M + H] 437.1664, found 437.1687.
N,N′-((butane-1,4-diylbis(oxy))bis(propane-3,1-diyl))bis(4-(trifluoromethyl)aniline) (18). Obtained according to method A from dioxadiamine 3 (0.5 mmol, 102 mg), 1-iodo-4-(trifluoromethyl)benzene (1.25 mmol, 340 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2–MeOH 100:1. Yield 61 mg (28%). 1H-NMR (400 MHz, CDCl3) δ 1.67–1.70 (m, 4H, CH2CH2CH2CH2), 1.94 (quintet, 4H, 3J = 5.9 Hz, OCH2CH2CH2N), 3.33 (t, 4H, 3J = 6.7 Hz, CH2N), 3.47–3.50 (m, 4H, OCH2), 3.57 (t, 4H, 3J = 5.7 Hz, OCH2CH2CH2N), 6.88 (d, 4H, 3Jobs = 8.4 Hz, H2, H2′ (Ph)), 7.46 (d, 4H, 3Jobs = 8.4 Hz, H3, H3′ (Ph)), NH protons were not unambiguously assigned. 13C-NMR (100.6 MHz, CDCl3) δ 26.6 (2C, CH2CH2CH2CH2), 29.0 (2C, OCH2CH2CH2N), 41.8 (2C, CH2N), 69.5 (2C, OCH2), 70.7 (2C, OCH2), 111.6 (4C, C2, C2′ (Ph)), 118.3 (q, 2C, 2JCF = 32.9 Hz, C4 (Ph)), 122.3 (q, 2C, 1JCF = 269.8 Hz, CF3 (Ph)), 126.5 (br. s, 4C, C3, C3′ (Ph)), 150.9 (2C, C1 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –61.48 (6F, CF3). MS (MALDI-TOF+): Calculated for C24H31F6N2O2 [M + H] 493.2290, found 493.2334.
N,N′-((butane-1,4-diylbis(oxy))bis(propane-3,1-diyl))bis(3-(trifluoromethyl)aniline) (19). Obtained according to method A from dioxadiamine 3 (0.5 mmol, 102 mg), 1-iodo-3-(trifluoromethyl)benzene (1.25 mmol, 340 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2. Yield 224 mg (91%). 1H-NMR (400 MHz, CDCl3) δ 1.67–1.70 (m, 4H, OCH2CH2CH2CH2O), 1.89 (quintet, 4H, 3J = 5.9 Hz, OCH2CH2CH2N), 3.24 (t, 4 H, 3J = 6.5 Hz, CH2NH), 3.45–3.48 (m, 4H, OCH2), 3.55 (t, 4H, 3J = 5.7 Hz, OCH2), 4.33 (br. s, 2H, NH), 6.73 (dd, 2H, 3J = 7.8 Hz, 4J = 1.9 Hz, H6 (Ph)), 6.77 (s, 2H, H2 (Ph)), 6.89 (d, 2H, 3J = 7.7 Hz, H4 (Ph)), 7.22 (t, 2H, 3Jobs = 7.8 Hz, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 26.6 (2C, CH2CH2CH2CH2), 29.1 (2C, OCH2CH2CH2NH), 42.0 (2C, CH2N), 69.5 (2C, OCH2), 70.8 (2C, OCH2), 108.5 (2C, C2 (Ph)), 113.2 (2C, C4 (Ph)), 115.6 (2 C, C6 (Ph)), 124.4 (q, 2C, 1JCF = 273.5 Hz, CF3), 129.5 (2C, C5 (Ph)), 131.4 (q, 2C, 2JCF = 31.0 Hz, C3 (Ph)), 148.7 (2 C, C1 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –62.86 (6F, CF3). MS (MALDI-TOF+): Calculated for C24H31F6N2O2 [M + H] 493.2290, found 493.2334.
4,4′-((((Oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(azandiyl))bis(2-(trifluoromethyl)-benzonitrile) (20). Obtained as one of several products according to method A from trioxadiamine 1 (0.5 mmol, 110 mg), 4-iodo-2-(trifluoromethyl)benzonitrile (1.25 mmol, 371 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (34 mg). Eluent CH2Cl2–MeOH 200:1. Yield 47 mg (17%). 1H-NMR (400 MHz, CDCl3) δ 1.88 (quintet, 4H, 3J = 6.1 Hz, CH2CH2CH2NH), 3.28 (q, 4H, 3J = 5.2 Hz, CH2N), 3.59–3.62 (m, 8H, OCH2), 3.66–3.69 (m, 4H, OCH2), 6.67 (dd, 1H, 3J = 8.7 Hz, 4J = 2.4 Hz, H6 (Ph)), 6.80 (d, 2H, 4J = 2.4 Hz, H2 (Ph)), 7.48 (d, 2H, 3J = 8.7 Hz, H5 (Ph)). NH protons were not unambiguously assigned. MS (MALDI-TOF+): Calculated for C26H29F6N4O3 [M + H] 559.214, found 559.227.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(3,5-bis(trifluoromethyl)aniline) (21). Obtained according to method B from trioxadiamine 1 (0.5 mmol, 110 mg), 1-bromo-3,5-di(trifluoromethyl)benzene (1 mmol, 293 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2–MeOH 200:1. Yield 257 mg (80%). 1H-NMR (400 MHz, CDCl3) δ 1.88 (quintet, 4H, 3J = 6.0 Hz, OCH2CH2CH2NH), 3.25 (q, 4H, 3J = 6.2 Hz, CH2N), 3.60–3.63 (m, 8H, OCH2), 3.69–3.72 (m, 4H, OCH2), 4.92 (br. s, 2H, NH), 6.91 (s, 4H, H2, H6 (Ph)), 7.08 (s, 2H, H4 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.3 (2C, OCH2CH2CH2NH), 42.2 (2C, CH2N), 70.0 (2C, OCH2), 70.1 (2C, OCH2), 70.4 (2C, OCH2), 109.5 (2C, C4 (Ph)), 111.6 (4C, C2, C6 (Ph)), 123.7 (q, 4C, 1JCF = 272.6 Hz, CF3), 132.1 (q, 4C, 2JCF = 32.4 Hz, C3, C5 (Ph)), 149.0 (2C, C1 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –63.19 (12F, CF3). MS (MALDI-TOF+): Calculated for C26H29F12N2O3 [M + H] 645.199, found 645.185.
N-(3,5-bis(trifluoromethyl)phenyl)-N-(3-(2-(2-(3-((3,5-bis(trifluoromethyl)phenyl)amino)- propoxy)ethoxy)ethoxy)propyl)-3,5-bis(trifluoromethyl)aniline (21a). Obtained as the second ptoduct in the synthesis of compound 21. Eluent CH2Cl2. Yield 30 mg (7%). 1H-NMR (400 MHz, CDCl3) δ 1.85–1.93 (m, 4H, OCH2CH2CH2N), 3.28 (t, 2H, 3J = 6.1 Hz, CH2NH), 3.49 (t, 2H, 3J = 6.1 Hz, OCH2), 3.60–3.63 (m, 6H, OCH2), 3.67–3.70 (m, 4H, OCH2), 3.96 (t, 2H, 3J = 6.1 Hz, CH2NPh2), 6.95 (s, 2H, H2, H6 (Ph)), 7.11 (s, 1H, H4 (Ph)), 7.46 (s, 4H, H2, H6 (Ph2)), 7.49 (s, 2H, H4 (Ph2)), NH proton was not unambiguously assigned. 13C-NMR (100.6 MHz, CDCl3) δ 27.8 (1C, OCH2CH2CH2N), 28.3 (1C, OCH2CH2CH2N), 42.5 (1C, CH2NHPh), 49.1 (1C, CH2NPh2), 67.3 (1C, OCH2), 70.2 (2C, OCH2), 70.3 (1C, OCH2), 70.4 (1C, OCH2), 70.6 (1C, OCH2), 109.8 (1C, C4 (Ph)), 111.8 (2C, C2, C6 (Ph)), 115.8 (2C, C4 (Ph2)), 120.6 (4C, C2, C6 (Ph2)), 123.0 (q, 6C, 1JCF = 273.1 Hz, CF3), 132.6 (q, 2C, 2JCF = 33.3 Hz, C3, C5 (Ph)), 133. 2 (q, 4C, 2JCF = 32.8 Hz, C3, C5 (Ph2)), 147.8 (2C, C1 (Ph2)), 148.9 (1C, C1 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –63.21 (6F, CF3), –63.23 (12F, CF3). MS (MALDI-TOF+): Calculated for C34H31F18N2O3 [M + H] 857.247, found 857.255.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(3-trifluoromethyl-2-fluoroaniline) (22). Obtained according to method B from trioxadiamine 1 (0.5 mmol, 110 mg), 1-bromo-2-fluoro-3-(trifluoromethyl)benzene (1 mmol, 243 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2–MeOH 200:1. Yield 177 mg (65%). 1H-NMR (400 MHz, CDCl3) δ 1.91 (quintet, 4H, 3J = 6.0 Hz, OCH2CH2CH2N), 3.26 (t, 4H, 3J = 6.3 Hz, CH2N), 3.59–3.62 (m, 8H, OCH2), 3.65–3.68 (m, 4H, OCH2), 4.55 (br. s, 2H, NH), 6.78–6.85 (m, 4H, H4, H6 (Ph)), 7.02 т (2H, 3J = 8.0 Hz, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.8 (2C, OCH2CH2CH2NH), 41.7 (2C, CH2N), 69.7 (2C, OCH2), 70.4 (2C, OCH2), 70.6 (2C, OCH2), 112.5 (q, 2C, 3JCF = 4.3 Hz, C4(Ph)), 115.0 (br. s, 2C, C6 (Ph)), 117.4 (qd, 2C, 2JCF = 33.0 Hz, 2JCF = 10.7 Hz, C3(Ph)), 120.3 (q, 2C, 1JCF = 272.0 Hz, CF3), 124.2 (d, 2C, 4JCF = 7.6 Hz C5 (Ph)), 137.7 (d, 2C, 2JCF = 10.7 Hz, C1 (Ph)), 148.1 (d, 2C, 1JCF = 248.0 Hz, C2 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –61.13 (d, 6F, JFF = 12.3 Hz, CF3); –138.43 (m, 2F, 2-F). MS (MALDI-TOF+): Calculated for C24H29F8N2O3 [M + H] 545.2050, found 545.2017.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(5-trifluromethyl-2-fluoroaniline) (23). Obtained according to method B from trioxadiamine 1 (0.5 mmol, 110 mg), 2-bromo-1-fluoro-4-(trifluoromethyl)benzene (1 mmol, 243 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2–MeOH 200:1. Yield 190 mg (70%). 1H-NMR (400 MHz, CDCl3) δ 1.92 (quintet, 4H, 3J = 6.0 Hz, 3J, OCH2CH2CH2NH), 3.27 (t, 4H, 3J = 6.4 Hz, CH2N), 3.59–3.62 (m, 8H, OCH2), 3.66–3.69 (m, 4H, OCH2), 4.61 (br. s, 2H, NH), 6.82–6.86 (m, 4H, H4, H6 (Ph)), 6.98 (dd, 2H, 3JHF = 11.0 Hz, 3JHH = 8.2 Hz, H3 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.8 (2C, OCH2CH2CH2NH), 41.4 (2C, CH2N), 69.7 (2C, OCH2), 70.4 (2C, OCH2), 70.6 (2C, OCH2), 108.1 (2C, C4 (Ph)), 113.0 (2C, C6 (Ph)), 114.2 (d, 2C, 2JCF = 20.1 Hz, C3 (Ph)), 124.2 (q, 2C, 1JCF = 271.5 Hz, CF3), 127.1 (q, 2C, 2JCF = 30.8 Hz, C5 (Ph)), 137.4 (2C, C1 (Ph)), 152.8 (d, 2C, 1JCF = 244.2 Hz, C2 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –62.11 (s, 6F, CF3); –131.82 (m, 2F, 2-F). MS (MALDI-TOF+): Calculated for C24H29F8N2O3 [M + H] 545.2050, found 545.2021.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(2-trifluoromethyl-6-fluoroaniline) (24). Obtained according to method B from trioxadiamine 1 (0.5 mmol, 110 mg), 2-bromo-1-fluoro-3-(trifluoromethyl)benzene (1 mmol, 243 mg) in the presence of Pd(dba)2 (23.2 mg) and BINAP (28.2 mg). Eluent CH2Cl2–MeOH 200:1. Yield 49 mg (18%). 1H-NMR (400 MHz, CDCl3) δ 1.86 (quintet, 4H, 3J = 6.2 Hz, OCH2CH2CH2N), 3.46 (td, 4H, 3JHH = 6.7 Hz, 4JHH = 4.5 Hz, CH2N), 3.56–3.60 (m, 8H, OCH2), 3.63–3.66 (m, 4H, OCH2), 4.14 (br. s, 2H, NH), 6.71 (td, 2 H, 3JHHobs = 8.2 Hz, 4JHF = 4.5 Hz, H3 (Ph)), 7.11 (dd, 2H, 3JHF = 12.7 Hz, 3JHH = 8.1 Hz, H5 (Ph)), 7.22 (t, 2H, 3J HH = 7.9 Hz, H4 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 30.4 (2 C, OCH2CH2CH2NH), 45.1 (d, 2C, 4JCF = 10.1 Hz, CH2N), 69.5 (2C, OCH2), 70.4 (2C, OCH2), 70.5 (2C, OCH2), 107.4 (q, 2C, 2JCF = 29.9 Hz, C2 (Ph)), 117.8 (d, 2C, 3JCF = 7.6 Hz, C4 (Ph)), 120.0 (d, 2C, 2JCF = 21.2 Hz, C5 (Ph)), 122.2 (br. s, 2C, C3 (Ph)), 124.4 (q, 2C, 1JCF = 271.8 Hz, CF3), 135.6 (d, 2C, 2JCF = 11.0 Hz, C1 (Ph)), 153.5 (d, 2C, 1JCF = 242.9 Hz, C6 (Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –61.08 (d, 6F, JFF = 12.3 Hz, CF3); –124.47 (m, 2F, 2-F). MS (MALDI-TOF+): Calculated for C24H26F7N2O3 [M – H2 – F] 523.1832, found 523.1878.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(2-trifluoromethyl-6-fluoroaniline) (24a). Obtained as the second product in the synthesis of compound 24. Eluent CH2Cl2–MeOH 50:1. Yield 13 mg (7%). 1H-NMR (400 MHz, CDCl3) δ 1.77 (a, 2H, 3J = 5.9 Hz, OCH2CH2CH2NH2), 1.88 (q, 2H, 3J = 5.7 Hz, OCH2CH2CH2NHPh), 3.30 (t, 2H, 3J = 5.9 Hz, CH2NH2), 3.42–3.64 (m, 14 H, CH2NPh, OCH2), 4.90 (br. s, 1H, NH), 6.80 (td, 1H, 3JHHobs = 7.8 Hz, 4JHF = 4.9 Hz, H4 (Ph)), 7.04 (ddd, 1H, 3JHF = 11.2 Hz, 3JHH = 7.8 Hz, 4JHH = 1.5 Hz, H5 (Ph)), 7.43 (d, 1H, 3J = 7.8 Hz, H5 (Ph)). NH2 were not unambiguously assigned. 13C-NMR (100.6 MHz, CDCl3) δ 29.3 (1C, OCH2CH2CH2NHPh), 31.1 (1C, OCH2CH2CH2NH2), 38.4 (1C, CH2NH2), 47.0 (1C, CH2NPh), 69.9 (1C, OCH2), 70.6 (2C, OCH2), 70.8 (2C, OCH2), 71.1 (1C, OCH2), 107.4 (q, 1C, 2JCF = 29.9 Hz, C2 (Ph)), 117.7 (d, 1C, 3JCF = 21.2 Hz, C5 (Ph)), 120.0 (d, 1C, 2JCF = 7.6 Hz, C4 (Ph)), 125.2 (1 C, C3 (Ph)), 123.7 (q, 1C, 1JCF = 270.5 Hz, CF3), 136.9 (d, 1C, 2JCF = 12.9 Hz, C1 (Ph)), 155.2 (d, 2C, 1JCF = 240.0 Hz, C6 (Ph)). MS (MALDI-TOF+): Calculated for C17H27F4N2O3 [M + H] 383.1958, found 383.1940.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(2-trifluoromethyl-3-fluoroaniline) (25). Obtained according to method B from trioxadiamine 1 (0.5 mmol, 110 mg), 1-bromo-2-(trifluoromethyl)-3-fluorobenzene (1 mmol, 243 mg) in the presence of Pd(dba)2 (23.2 mg) and BINAP (28.2 mg). Eluent CH2Cl2. Yield 46 mg (17%). 1H-NMR (400 MHz, CDCl3) δ 1.92 (quintet, 4H, 3J = 5.8 Hz, OCH2CH2CH2N), 3.24 (q, 4H, 3J = 5.7 Hz, CH2N), 3.59–3.62 (m, 8H, OCH2), 3.64–3.67 (m, 4H, OCH2), 5.22 (br. s, 2H, NH), 6.37 (dd, 2H, 3JHH = 8.5 Hz, 3JHF = 11.3 Hz, H4 (Ph)), 6.37 (d, 2H, 3J = 8.5 Hz, H6 (Ph)), 7.23 (td, 2H, 3JHH = 8.2 Hz, 4JHF = 6.3 Hz, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.7 (2C, OCH2CH2CH2N), 42.3 (2C, CH2N), 68.8 (2C, OCH2), 70.4 (2C, OCH2), 70.5 (2C, OCH2), 101.2 (q, 2C, 2JCF = 30.0 Hz, C2 (Ph)), 103.5 (d, 2C, 2JCF = 23.2 Hz, C4 (Ph)), 107.3 (2C, C6 (Ph)), 124.6 (q, 2C, 1JCF = 273.6 Hz, CF3), 133.5 (d, 2C, 3JCF = 11.8 Hz, C5 (Ph)), 147.5 (2C, C1 (Ph)), 161.5 (d, 2C, 1JCF = 253.4 Hz, C3 (Ph)). MS (MALDI-TOF+): Calculated for C24H29F8N2O3 [M + H] 545.205, found 545.196.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(2-trifluoromethyl-5-fluoroaniline) (26) Obtained according to method B from trioxadiamine 1 (0.5 mmol, 110 mg), 1-bromo-2-(trifluoromethyl)-5-fluorobenzene (1 mmol, 243 mg) in the presence of Pd(dba)2 (23.2 mg) and BINAP (28.2 mg). Eluent CH2Cl2. Yield 79 mg (29%). 1H-NMR (400 MHz, CDCl3) δ 1.92 (quintet, 4H, 3J = 5.9 Hz, OCH2CH2CH2N), 3.23 (q, 4H, 3J = 5.7 Hz, CH2N), 3.59–3.62 (m, 8H, OCH2), 3.65–3.68 (m, 4H, OCH2), 5.02 (br. s, 2H, NH), 6.31–6.38 (m, 4H, H4, H6 (Ph)), 7.36 (dd, 2H, 3JHH = 8.6 Hz, 4JHF = 6.3 Hz, H3 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.6 (2C, OCH2CH2CH2N), 41.8 (2C, CH2N), 69.7 (2C, OCH2), 70.4 (2C, OCH2), 70.5 (2C, OCH2), 98.4 (d, 2C, 2JCF = 26.9 Hz, C6 (Ph)), 102.1 (d, 2C, 2JCF = 22.8 Hz, C4 (Ph)), 109.3 (qd, 2C, 2JCF = 30.0 Hz, 4JCF = 2.2 Hz, C2 (Ph)), 124.9 (q, 2C, 1JCF = 270.0 Hz, CF3), 128.6 (dq, 2C, 3JCF = 11.4 Hz, 3JCF = 5.7 Hz, C3(Ph)), 147,9 (dq, 2C, 3JCF = 12.0 Hz, 3JCF =1.4 Hz, C1(Ph)), 166.2 (dq, 2C, 1JCF = 248.1 Hz, 5JCF = 1.0 Hz, C5(Ph)). 19F-NMR (376.4 MHz, CDCl3) δ –61.98 (d, 6F, JFF = 12.3, CF3); –107.62 (br. s, 2F, 2-F). MS (MALDI-TOF+): Calculated for C24H29F8N2O3 [M + H] 545.205, found 545.192.
N-(3-(2-(2-(3-aminopropoxy)ethoxy)ethoxy)propyl)-2-trifluoromethyl-5-fluoroaniline (26a). Obtained as the second product in the synthesis of compound 26. Eluent CH2Cl2–MeOH 50:1. Yield 23 mg (12%). 1H-NMR (400 MHz, CDCl3) δ 1.81 (quintet, 2H, 3J = 5.8 Hz, OCH2CH2CH2NH2), 1.90 (quintet, 2H, 3J = 5.5 Hz, OCH2CH2CH2NHPh), 3.24 (t, 2H, 3J = 5.5 Hz, CH2NH2), 3.45–3.59 (m, 6H, OCH2, CH2NPh), 3.60 (s, 4H, OCH2), 3.63–3.67 (m, 4H, OCH2), 5.02 (br. s, 2H, NH), 6.24 (ddd, 1H, 3JHH = 8.6 Hz, 3JHF = 8.6 Hz, 4JHH = 2.0 Hz, H4 (Ph)), 6.33 (dd, 1H, 3JHF = 12.2 Hz, 4JHH = 2.0 Hz, H6 (Ph)), 7.28 (dd, 2H, 3JHH = 8.2 Hz, 4JHF = 6.3 Hz, H3 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.6 (1C, OCH2CH2CH2N), 29.7 (1C, OCH2CH2CH2N), 36.8 (1C, CH2NH2) 41.7 (1C, CH2N), 68.5 (1C, OCH2), 70.1 (1C, OCH2), 70.5 (1C, OCH2), 70.6 (1C, OCH2), 70.7 (1C, OCH2), 71.2 (1C, OCH2), 98.2 (d, 1C, 2JCF = 26.9 Hz, C6 (Ph)), 101.2 (d, 1C, 2JCF = 22.8 Hz, C4 (Ph)), 129.2 (d, 1C, 3JCF = 11.6 Hz, C3(Ph)), 150.7 (br.s, 1C, C1(Ph)), 165.6 (d, 2C, 1JCF = 247.9 Hz, C5(Ph)), quaternary carbon atoms C2 and CF3 were not unambiguously assigned. MS (MALDI-TOF+): Calculated for C17H27F4N2O3 [M + H] 383.1958, found 383.1977.
4,4′-((((Oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(azanediyl))dibenzonitrile (27). Obtained according to method A from trioxadiamine 1 (0.5 mmol, 110 mg), 4-iodobenzonitrile (1.25 mmol, 286 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (33 mg). Eluent CH2Cl2/MeOH 200:1 Yield 192 mg (91%). 1H-NMR (400 MHz, CDCl3) δ 1.86 (quintet, 4H, 3J = 6.2 Hz, OCHCH2CH2N), 3.23 (t, 4H, 3J = 6.3 Hz, CH2N), 3.56–3.59 (m, 8H, OCH2), 3.64–3.66 (m, 4H, OCH2), 5.02 (br. s, 2H, NH), 6.53 (d, 4H, 3Jobs = 8.8 Hz, H2, H2′(Ph)), 7.36 (d, 4H, 3Jobs = 8.8 Hz, H3, H3′(Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.1 (2C, OCH2CH2CH2N), 41.1 (2C, CH2N), 69.3 (2C, OCH2), 69.7 (2C, OCH2), 70.1 (2C, OCH2), 97.7 (2C, C1(Ph)), 111.8 (4C, C2, C2′(Ph)), 120.3 (2C, CN (Ph)), 133.2 (4C, C3, C3′ (Ph)), 152.0 (2C, C4 (Ph)). MS (MALDI-TOF+): Calculated for C24H31N4O3 [M + H] 423.2396, found 423.2425.
3,3′-((((Oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(azanediyl))dibenzonitrile (28). Obtained according to method A from trioxadiamine 1 (0.5 mmol, 110 mg), 3-iodobenzonitrile (1.25 mmol, 286 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (33 mg). Eluent CH2Cl2/MeOH 50:1 Yield 165 mg (78%). 1H-NMR (400 MHz, CDCl3) δ 1.83 (quintet, 4H, 3J = 5.9 Hz, CH2CH2CH2N), 3.15 (t, 4H, 3J = 6.3 Hz, CH2N), 3.55–3.58 (m, 8H, OCH2), 3.64–3.67 (m, 4H, OCH2), 6.71–6.73 (m, 4H, H2, H6(Ph)), 6.84 (d, 2H, 3J = 7.8 Hz, H4(Ph)), 7.13 (t, 2H, 3J = 7.8 Hz, H5 (Ph)). NH protons were not unambiguously assigned. 13C-NMR (100.6 MHz, CDCl3) δ 28.1 (2C, CH2CH2CH2N), 41.2 (2C, CH2N), 69.4 (2C, OCH2), 69.7 (2C, OCH2), 70.1 (2C, OCH2), 112.4 (2C, C3(Ph)), 114.3 (2C, CH(Ph)), 116.5 (2C, CH(Ph)), 119.3 (2C, CN), 119.5 (2C, CH(Ph)), 129.4 (2C, C5(Ph)), 148.3 (2C, C1(Ph)). MS (MALDI-TOF+): Calculated for C24H31N4O3 [M + H] 423.2396, found 423.2362.
2,2′-((((Oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(azanediyl))dibenzonitrile (29). Obtained according to method B from trioxadiamine 1 (0.5 mmol, 110 mg), 2-bromobenzonitrile (1 mmol, 182 mg) in the presence of Pd(dba)2 (11.6 mg) and BINAP (15.7 mg). Eluent CH2Cl2/MeOH 100:1 Yield 198 mg (04%). 1H-NMR (400 MHz, CDCl3) δ 1.89 (quintet, 4H, 3J = 6.0 Hz, CH2CH2CH2N), 3.28 (q, 4H, 3J = 6.0 Hz, CH2N), 3.57–3.61 (m, 8H, OCH2), 3.64–3.67 (m, 4H, OCH2), 5.03 (br. s, 2H, NH), 6.59 (t, 2H, 3Jobs = 7.2 Hz, H4 (Ph)), 6.63 (d, 2H, 3J = 8.7 Hz, H6(Ph)), 7.31–7.35 (m, 4H, H3, H5(Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 27.5 (2C, CH2CH2CH2N), 41.0 (2C, CH2N), 69.1 (2C, OCH2), 70.0 (4C, OCH2), 95.0 (2C, C2(Ph)), 110.0 (2C, CH(Ph)), 115.7 (2C, CH(Ph)), 117.7 (2C, CN), 132.4 (2C, CH(Ph)), 133.8 (2C, CH(Ph)), 150.1 (2C, C1(Ph)). MS (MALDI-TOF+): Calculated for C24H31N4O3 [M + H] 423.2396, found 423.2375.
2-((3-(2-(2-(3-Aminorpopxy)ethoxy)ethoxy)propyl)amino)benzonitrile (29a). Obtained as the second product in the synthesis of compound 29 according to method A using trioxadiamine 1 (0.5 mmol, 110 mg), 2-iodobenzonitrile (1.25 mmol, 286 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (33 mg). Eluent CH2Cl2/MeOH 50:1 Yield 61 mg (38%). 1H-NMR (400 MHz, CDCl3) δ 1.77 (quintet, 2H, 3J = 5.9 Hz, OCH2CH2CH2NH2), 1.89 (quintet, 2H, 3J = 5.8 Hz, OCH2CH2CH2NPh), 3.28 (t, 2H, 3J = 6.2 Hz, CH2NH2), 3.42 (q, 2H, 3J = 6.1 Hz CH2NPh), 3.58–3.63 (m, 8H, OCH2), 3.65–3.68 (m, 2H, OCH2), 3.69–3.72 (m, 2H, OCH2), 6.61–6.65 (m, 2H, H4, H6 (Ph)), 7.34–7.38 (m, H3, H5 (Ph)). MS (MALDI-TOF+): Calculated for C17H28N3O3 [M + H] 322.213, found 322.205.
1,1′-((((Oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(azanediyl))bis(4,1-phenylene))bis(ethan-1-one) (30). Obtained according to method A using trioxadiamine 1 (0.5 mmol, 110 mg), 4-iodoacetophenone (1.25 mmol, 308 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (33 mg). Eluent CH2Cl2/MeOH 100:1 Yield 194 mg (85%). 1H-NMR (400 MHz, CDCl3) δ 1.86 (quintet, 4H, 3J = 6.0 Hz, CH2CH2CH2N), 2.45 (s, 6H, CH3), 3.26 (t, 4H, 3J = 6.4 Hz, CH2N), 3.56–3.59 (m, 8H, OCH2), 3.64–3.67 (m, 4H, OCH2), 5.07 (br. s, 2H, NH), 6.52 (d, 4H, 3Jobs = 8.8 Hz, H2, H2′ (Ph)), 7.76 (d, 4H, 3Jobs = 8.8 Hz, H3, H3′ (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 25.9 (2C, CH3), 28.5 (2C, CH2CH2CH2NH), 41.4 (2C, CH2N), 69.6 (2C, OCH2), 70.1 (2C, OCH2), 70.2 (2C, OCH2), 111.3 (4C, C2, C2′ (Ph)), 126.2 (2C, C4 (Ph)), 130.7 (4C, C3, C3′ (Ph)), 152.3 (2C, C1, (Ph)), 196.1 (2C, CO). MS (MALDI-TOF+): Calculated for C26H37N2O5 [M + H] 457.2702, found 457.2680.
1,1′-((((Oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(azanediyl))bis(3,1-phenylene))bis(ethan-1-one) (31). Obtained according to method A using trioxadiamine 1 (0.5 mmol, 110 mg), 3-iodoacetophenone (1.25 mmol, 308 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (33 mg). Eluent CH2Cl2/MeOH 100:1 Yield 103 mg (45%). 1H-NMR (400 MHz, CDCl3) δ 1.89 (quintet, 4H, 3J = 6.0 Hz, CH2CH2CH2NH), 2.55 (s, 6H, CH3) 3.28 (t, 4H, 3J = 6.4 Hz, CH2N), 3.59–3.62 (m, 8H, OCH2), 3.67–3.71 (m, 4H, OCH2), 6.92 (d, 2H, 3J = 8.8 Hz, H6(Ph)), 7.21–7.32 (m, 6H, H2, H4, H5 (Ph)), NH were not unambiguously assigned. 13C-NMR (100.6 MHz, CDCl3) δ 26.3 (2C, CH3), 27.9 (2C, CH2CH2CH2NH), 42.7 (2C, CH2N), 69.3 (2C, OCH2), 69.8 (2C, OCH2), 70.2 (2C, OCH2), 112.3 (2C, CH(Ph)), 118.4 (4C, CH(Ph)), 129.0 (2C, C5(Ph)), 137.7 (2C, C3(Ph)), 146.8 (2C, C1(Ph)), 198.1 (2C, CO). MS (MALDI-TOF+): Calculated for C26H37N2O5 [M + H] 457.2702, found 457.2672.
((((Oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(azanediyl))bis(3,1-phenylene))bis(phenylmethanone) (32). Obtained according to method A using trioxadiamine 1 (0.5 mmol, 110 mg), 4-iodobenzophenone (1.25 mmol, 385 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (33 mg). Eluent CH2Cl2/MeOH 100:1 Yield 206 mg (71%). 1H-NMR (400 MHz, CDCl3) δ 1.86 (quintet, 4H, 3J = 6.0 Hz, CH2CH2CH2N), 3.27 (t, 4H, 3J = 6.4 Hz, CH2N), 3.56–3.60 (m, 8H, OCH2), 3.64–3.67 (m, 4H, OCH2), 5.07 (br. s, 2H, NH), 6.64 (d, 4H, 3Jobs = 8.8 Hz, H2, H2′ (Ph)), 7.41 (t, 2H, 3Jobs = 7.6 Hz, H4 (Ph′)), 7.48 (t, 4H, 3Jobs = 7.5 Hz, H3, H3′ (Ph′)), 7.66–7.71 (m, 8H, H3, H3′ (Ph), H2, H2′ (Ph′)). 13C-NMR (100.6 MHz, CDCl3) δ 28.3 (2C, CH2CH2CH2NH), 40.9 (2C, CH2N), 69.2 (2C, OCH2), 69.8 (2C, OCH2), 70.1 (2C, OCH2), 110.8 (4C, C2, C2′ (Ph)), 124.9 (2C, C4 (Ph′)), 127.6 (4C, CH (Ar)), 129.0 (4C, CH (Ar)), 130.7 (2C, C4 (Ph)), 132.6 (4C, CH (Ar)), 138.9 (2C, C1 (Ph′)), 152.2 (2C, C1 (Ph)), 194.7 (2C, CO). MS (MALDI-TOF+): Calculated for C36H41N2O5 [M + H] 581.3015, found 581.3044.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis ([1,1′-biphenyl]-4-amine)) (33). Obtained according to method A using trioxadiamine 1 (0.5 mmol, 110 mg), 4-iodobiphenyl (1.25 mmol, 350 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (33 mg). Eluent CH2Cl2/MeOH 200:1 Yield 170 mg (65%). 1H-NMR (400 MHz, CDCl3) δ 1.93 (quintet, 4H, 3J = 6.2 Hz, CH2CH2CH2NH), 3.29 (t, 4H, 3J = 6.6 Hz, CH2N), 3.62–3.66 (m, 8H, OCH2), 3.70–3.73 (m, 4H, OCH2), 3.96 (br. s, 2H, NH), 6.68 (d, 4H, 3Jobs = 8.6 Hz, H2, H2′ (Ph)), 7.27 (t, 2H, 3J = 7.6 Hz, H4 (Ph′)), 7.41 (t, 4H, 3Jobs = 7.6 Hz, H3, H3′ (Ph′)), 7.46 (d, 4H, 3Jobs = 8.6 Hz, H3, H3′ (Ph)), 7.66 (d, 4H, 3Jobs = 8.6 Hz, H2, H2′ (Ph′)). 13C-NMR (100.6 MHz, CDCl3) δ 28.7 (2C, CH2CH2CH2NH), 41.4 (2C, CH2N), 69.4 (2C, OCH2), 69.9 (2C, OCH2), 70.3 (2C, OCH2), 112.5 (4C, C2, C2′ (Ph)), 125.6 (2C, C4 (Ph′)), 125.8 (4C, CH (Ar)), 127.5 (4C, CH (Ar)), 128.3 (4C, CH (Ar)), 128.3 (2C, C4 (Ph)), 140.9 (2C, C1 (Ph′)), 147.6 (2C, C1 (Ph)). MS (MALDI-TOF+): Calculated for C34H41N2O3 [M + H] 525.3117, found 525.3166.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis([1,1′-biphenyl]-3-amine)) (34). Obtained according to method B from trioxadiamine 1 (0.5 mmol, 110 mg), 3-bromobiphenyl (1 mmol, 233 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2/MeOH 200:1 Yield 207 mg (79%). 1H-NMR (400 MHz, CDCl3) δ 1.93 (quintet, 4H, 3J = 6.2 Hz, CH2CH2CH2N), 3.29 (t, 4H, 3J = 6.6 Hz, CH2N), 3.62–3.66 (m, 8H, OCH2), 3.70–3.73 (m, 4H, OCH2), 4.17 (br. s, 2H, NH), 6.61 (d, 2H, 3J = 8.0 Hz, H6 (Ph)), 6.82 (s, 2H, H2 (Ph)), 6.93 (d, 2H, 3J = 7.8 Hz, H4 (Ph)), 7.25 (t, 2H, 3J = 7.3 Hz, H5 (Ph)), 7.34 (t, 4H, 3Jobs = 7.3 Hz, H3, H3′ (Ph′)), 7.43 (t, 2H, 3Jobs = 7.8 Hz, H4 (Ph′)), 7.60 (d, 4H, 3Jobs = 7.3 Hz, H2, H2′ (Ph′)). 13C-NMR (100.6 MHz, CDCl3) δ 28.7 (2C, CH2CH2CH2N), 41.4 (2C, CH2N), 69.4 (2C, OCH2), 70.0 (2C, OCH2), 70.3 (2C, OCH2), 111.1 (2C, CH (Ph)), 111.4 (2C, CH (Ph)), 115.8 (2C, CH (Ph)), 126.7 (2C, C4 (Ph′)), 126.8 (4C, CH (Ph′)), 128.2 (4C, CH (Ph′)), 129.2 (2C, C5 (Ph)), 141.5 (2C, C (Ar)), 141.9 (2C, C (Ar)), 148.5 (2C, C1 (Ph)). MS (MALDI-TOF+): Calculated for C34H41N2O3 [M + H] 525.3117, found 525.3090.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(4-methoxyaniline) (35). Obtained according to method A using trioxadiamine 1 (0.5 mmol, 110 mg), 4-iodoanisole (1.25 mmol, 293 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (33 mg). Eluent CH2Cl2/MeOH 50:1 Yield 93 mg (43%). 1H-NMR (400 MHz, CDCl3) δ 1.86 (quintet, 4H, 3J = 6.3 Hz, CH2CH2CH2N), 3.17 (t, 4H, 3J = 6.6 Hz, CH2N), 3.57–3.60 (m, 8H, OCH2), 3.65–3.68 (m, 4H, OCH2), 3.72 (s, 6H, OCH3), 6.58 (d, 4H, 3Jobs = 8.8 Hz, H2, H2′ (Ph)), 6.76 (d, 4H, 3Jobs = 8.8 Hz, H3, H3′ (Ph)), NH protons were not unambiguously assigned. 13C-NMR (100.6 MHz, CDCl3) δ 29.0 (2C, CH2CH2CH2NH), 42.9 (2C, CH2N), 55.7 (2C, OCH3), 69.7 (2C, OCH2), 70.1 (2C, OCH), 70.5 (2C, OCH2), 114.2 (4C, C2, C2′ (Ph)), 114.7 (4C, C3, C3′ (Ph)), 142.3 (2C, C1 (Ph)), 152.0 (2C, C4 (Ph)). MS (MALDI-TOF+): Calculated for C24H37N2O5 [M + H] 433.2702, found 433.2681.
4-Methoxy-N-(4-methoxyphenyl)-N-(3-(2-(2-(3-((4-methoxyphenyl)amino)propoxy)ethoxy)ethoxy)propyl)aniline (35a). Obtained as the second product in the synthesis of compound 35 according to method B using trioxadiamine 1 (0.5 mmol, 110 mg), 4-bromoanisole (1 mmol, 187 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2/MeOH 100:1 Yield 11 mg (6%). 1H-NMR (400 MHz, CDCl3) δ 1.83 (quintet, 2H, 3J = 6.8 Hz, CH2CH2CH2NPh), 1.94 (quintet, 2H, 3J = 6.1 Hz, CH2CH2CH2NPh2), 3.26 (t, 2H, 3J = 6.6 Hz, CH2NPh), 3.49 (t, 2H, 3J = 6.1 Hz, CH2O), 3.52–3.57 (m, 2H, OCH2), 3.62–3.69 (m, 10H, OCH2, CH2NPh2), 3.74 c (3H, OCH3), 3.77 с (6H, OCH3), 4.68 (br. s, 1H, NH), 6.78–6.82 (m, 6H, H(Ph)), 6.86–6.91 (m, 6H, H(Ph)). MS (MALDI-TOF+): Calculated for C31H43N2O6 [M + H] 539.312, found 539.331.
N,N′-(((oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(3-methoxyaniline) (36). Obtained according to method B using trioxadiamine 1 (0.5 mmol, 110 mg), 3-bromoanisole (1 mmol, 187 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2/MeOH 100:1 Yield 158 mg (73%). 1H-NMR (400 MHz, CDCl3) δ 1.87 (quintet, 4H, 3J = 6.1 Hz, CH2CH2CH2NH), 3.21 (t, 4H, 3J = 6.5 Hz, CH2N), 3.57–3.62 (m, 8H, OCH2), 3.66–3.69 (m, 4H, OCH2), 3.76 c (6H, OCH3), 4.09 (br. s, 2H, NH), 6.15 (t, 2H, 4J = 2.2 Hz, H2 (Ph)), 6.22 (dd, 2H, 3J = 8.1 Hz, 4J = 2.2 Hz, H6 (Ph)), 6.25 (dd, 2H, 3J = 8.1 Hz, 4J = 2.2 Hz, H4 (Ph)), 7.06 (t, 2H, 3J = 8.1 Hz, H5 (Ph)). 13C-NMR (100.6 MHz, CDCl3) δ 28.9 (2C, CH2CH2CH2NH), 41.6 (2C, CH2N), 54.9 (2C, OCH3), 69.6 (2C, OCH2), 70.1 (2C, OCH2), 70.5 (2C, OCH2), 98.5 (2C, C2(Ph)), 101.9 (2C, C6 (Ph)), 105.8 (2C, C4 (Ph)), 129.8 (2C, C5 (Ph)), 149.9 (2C, C1 (Ph)), 160.8 (2C, C3 (Ph)). MS (MALDI-TOF+): Calculated for C24H37N2O5 [M + H] 433.2702, found 433.2676.
N-(3-(2-(2-(3-aminopropoxy)ethoxy)ethoxy)propyl)-3-methoxyaniline (36a). Obtained as the second product in the synthesis of compound 36 according to method A using trioxadiamine 1 (0.5 mmol, 110 mg), 3-iodoanisole (1.25 mmol, 293 mg) in the presence of CuI (19 mg) and 2-isobutyrylcyclohexanone (33 mg). Eluent CH2Cl2/MeOH 20:1 Yield 28 mg (17%). 1H-NMR (400 MHz, CDCl3) δ 1.76 (quintet, 2H, 3J = 6.0 Hz, CH2CH2CH2NH2), 1.87 (quintet, 2H, 3J = 6.2 Hz, CH2CH2CH2NPh), 3.19 (t, 2H, 3J = 6.5 Hz, CH2NH2), 3.39 (q, 2H, 3J = 6.2 Hz, CH2N), 3.56–3.59 (m, 8H, OCH2), 3.66–3.68 (m, 4H, OCH2), 3.76 c (3H, OCH3), 5.28 (br. s, 1H, NH), 6.18 (t, 1H, 4J = 2.2 Hz, H2 (Ph)), 6.23–6.26 (m, 2H, H4, H6 (Ph)), 7.05 (t, 2H, 3J = 8.0 Hz, H5 (Ph)). NH2 were not unambiguously assigned. 13C-NMR (100.6 MHz, CDCl3) δ 28.4 (1C, OCH2CH2CH2N), 28.6 (1C, OCH2CH2CH2N), 36.6 (1C, CH2NH2), 41.3 (1C, CH2NHPh), 55.0 (1C, OCH3), 69.7 (1C, OCH2), 70.0 (2C, OCH2), 70.1 (2C, OCH2), 70.4 (1C, OCH2), 99.5 (1C, C2(Ph)), 103.0 (1C, C6 (Ph)), 106.7 (1C, C4 (Ph)), 129.9 (1C, C5 (Ph)), 148.4 (1C, C1 (Ph)), 161.4 (1C, C3 (Ph)). MS (MALDI-TOF+): Calculated for C17H31N2O4 [M + H] 327.2284, found 327.2261.
(((((Oxybis(ethane-2,1-diyl))bis(oxy))bis(propane-3,1-diyl))bis(azanediyl))bis(3,1-phenylene))bis(phenylmethanone) (37). Obtained according to method B using trioxadiamine 1 (0.5 mmol, 110 mg), 3-bromobenzophenone (1 mmol, 187 mg) in the presence of Pd(dba)2 (2.9 mg) and BINAP (4.7 mg). Eluent CH2Cl2/MeOH 200:1 Yield 238 mg (82%). 1H-NMR (400 MHz, CDCl3) δ 1.89 (quintet, 4H, 3J = 6.1 Hz, CH2CH2CH2N), 3.25 (t, 4H, 3J = 6.4 Hz, CH2N), 3.57–3.60 (m, 8H, OCH2), 3.63–3.66 (m, 4H, OCH2), 6.81 (dd, 2H, 3J = 8.0 Hz, 4J = 2.2 Hz, H6 (Ph)), 7.00–7.04 (m, 4H, H4, H6 (Ph)), 7.21 (t, 2H, 3Jobs = 7.8 Hz, H5(Ph)), 7.44 (t, 4H, 3Jobs = 7.8 Hz, H3, H3′ (Ph′))), 7.65 (t, 2H, 3J = 7.8 Hz, H4 (Ph′)), 7.88 (d, 4H, 3Jobs = 7.1 Hz, H2, H2′ (Ph′)). 13C-NMR (100.6 MHz, CDCl3) δ 28.3 (2C, OCH2CH2CH2NH), 41.7 (2C, CH2N), 69.4 (2C, OCH2), 69.8 (2C, OCH2), 70.2 (2C, OCH2), 113.1 (2C, C6 (Ph)), 116.6 (2C, C4 (Ph)), 119.1 (2C, C2 (Ph)), 127.7 (4C, C3, C3′ (Ph′)), 128.4 (2C, C5 (Ph)), 129.6 (4C, C2, C2′ (Ph′)), 131.8 (2C, C4 (Ph′)), 137.5 (2C, C1 (Ph′) or C3 (Ph)), 138.1 (2C, C3 (Ph) or C1 (Ph′)), 147.9 (2C, C1 (Ph)), 196.8 (2C, CO). MS (MALDI-TOF+): Calculated for C36H41N2O5 [M + H] 581.3015, found 581.3070.