Exploring the Scope of Macrocyclic “Shoe-last” Templates in the Mechanochemical Synthesis of RHO Topology Zeolitic Imidazolate Frameworks (ZIFs)
Abstract
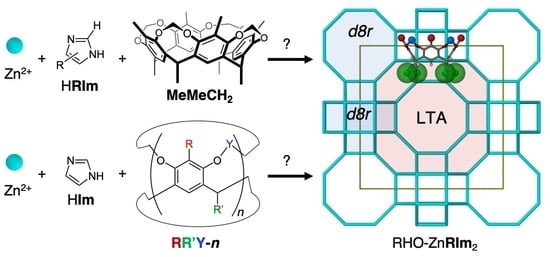
:1. Introduction
2. Results and Discussion
2.1. Accelerated Synthesis of RHO-Zn16(Cl2Im)32 and RHO-ZnBIm2 Using the MeMeCH2 Template
2.2. Exploring Different Cavitand Templates in the Synthesis of (Template)x@RHO-ZnIm2 Materials
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tian, Y.; Cai, C.; Ji, Y.; You, X.; Peng, S.; Lee, G. [Co5(Im)10⋅2MB]∞: A Metal-Organic Open-Framework with Zeolite-Like Topology. Angew. Chem. Int. Ed. 2002, 41, 1384–1386. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like Metal-Organic Frameworks (ZMOFs): Design, Synthesis, and Properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.X.; Wang, F.; Zhang, J. Design and Synthesis of Multifunctional Metal-Organic Zeolites. Chem. Soc. Rev. 2018, 47, 2130–2144. [Google Scholar] [CrossRef]
- Wu, T.; Bu, X.; Zhang, J.; Feng, P. New Zeolitic Imidazolate Frameworks: From Unprecedented Assembly of Cubic Clusters to Ordered Cooperative Organization of Complementary Ligands. Chem. Mater. 2008, 20, 7377–7382. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, T.; Zhou, C.; Chen, S.; Feng, P.; Bu, X. Zeolitic Boron Imidazolate Frameworks. Angew. Chem. Int. Ed. 2009, 48, 2542–2545. [Google Scholar] [CrossRef] [Green Version]
- O’Keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The Reticular Chemistry Structure Resource (RCSR) Database of, and Symbols for, Crystal Nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, S.M.; Boyd, P.G.; Sarkisov, L.; Smit, B. Improving the Mechanical Stability of Metal-Organic Frameworks Using Chemical Caryatids. ACS Cent. Sci. 2018, 4, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Lee, J.; Kim, J. Compositions and Structures of Zeolitic Imidazolate Frameworks. Isr. J. Chem. 2018, 58, 1075–1088. [Google Scholar] [CrossRef]
- Ramirez, J.R.; Yang, H.; Kane, C.M.; Ley, A.N.; Holman, K.T. Reproducible Synthesis and High Porosity of Mer-Zn(Im)2 (ZIF-10): Exploitation of an Apparent Double-Eight Ring Template. J. Am. Chem. Soc. 2016, 138, 12017–12020. [Google Scholar] [CrossRef]
- Friščić, T.; Halasz, I.; Beldon, P.J.; Belenguer, A.M.; Adams, F.; Kimber, S.A.J.; Honkimäki, V.; Dinnebier, R.E. Real-Time and in Situ Monitoring of Mechanochemical Milling Reactions. Nat. Chem. 2013, 5, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Akimbekov, Z.; Katsenis, A.D.; Nagabhushana, G.P.; Ayoub, G.; Arhangelskis, M.; Morris, A.J.; Friščić, T.; Navrotsky, A. Experimental and Theoretical Evaluation of the Stability of True MOF Polymorphs Explains Their Mechanochemical Interconversions. J. Am. Chem. Soc. 2017, 139, 7952–7957. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.Q.; Yao, S.Y.; Gu, D.; Cui, K.H.; Guo, D.W.; Zhang, G.; Chen, Z.X.; Zhao, D.Y. Cadmium Imidazolate Frameworks with Polymorphism, High Thermal Stability, and a Large Surface Area. Chem. Eur. J. 2010, 16, 1137–1141. [Google Scholar] [CrossRef]
- Morris, W.; Leung, B.; Furukawa, H.; Yaghi, O.K.; He, N.; Hayashi, H.; Houndonougbo, Y.; Asta, M.; Laird, B.B.; Yaghi, O.M. A Combined Experimental-Computational Investigation of Carbon Dioxide Capture in a Series of Isoreticular Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2010, 132, 11006–11008. [Google Scholar] [CrossRef]
- Banerjee, R.; Furukawa, H.; Britt, D.; Knobler, C.; O’Keeffe, M.; Yaghi, O.M. Control of Pore Size and Functionality in Isoreticular Zeolitic Imidazolate Frameworks and Their Carbon Dioxide Selective Capture Properties. J. Am. Chem. Soc. 2009, 131, 3875–3877. [Google Scholar] [CrossRef]
- Tian, Y.-Q.Q.; Cai, C.-X.X.; Ren, X.-M.M.; Duan, C.-Y.Y.; Xu, Y.; Gao, S.; You, X.-Z.Z. The Silica-Like Extended Polymorphism of Cobalt(II) Imidazolate Three-Dimensional Frameworks: X-Ray Single-Crystal Structures and Magnetic Properties. Chem. Eur. J. 2003, 9, 5673–5685. [Google Scholar] [CrossRef]
- Tian, Y.Q.; Zhao, Y.M.; Chen, Z.X.; Zhang, G.N.; Weng, L.H.; Zhao, D.Y. Design and Generation of Extended Zeolitic Metal-Organic Frameworks (ZMOFs): Synthesis and Crystal Structures of Zinc(II) Imidazolate Polymers with Zeolitic Topologies. Chem. Eur. J. 2007, 13, 4146–4154. [Google Scholar] [CrossRef]
- Shi, Q.; Kang, X.; Shi, F.N.; Dong, J. Zn10(Im)20·4DBF: An Unprecedented 10-Nodal Zeolitic Topology with a 10-MR Channel and 10 Crystallographically Independent Zn Atoms. Chem. Commun. 2015, 51, 1131–1134. [Google Scholar] [CrossRef]
- Shi, Q.; Xu, W.J.; Huang, R.K.; Zhang, W.X.; Li, Y.; Wang, P.; Shi, F.N.; Li, L.; Li, J.; Dong, J. Zeolite CAN and AFI-Type Zeolitic Imidazolate Frameworks with Large 12-Membered Ring Pore Openings Synthesized Using Bulky Amides as Structure-Directing Agents. J. Am. Chem. Soc. 2016, 138, 16232–16235. [Google Scholar] [CrossRef]
- Lalonde, M.B.; Mondloch, J.E.; Deria, P.; Sarjeant, A.A.; Al-Juaid, S.S.; Osman, O.I.; Farha, O.K.; Hupp, J.T. Selective Solvent-Assisted Linker Exchange (SALE) in a Series of Zeolitic Imidazolate Frameworks. Inorg. Chem. 2015, 54, 7142–7144. [Google Scholar] [CrossRef]
- Fei, H.; Cahill, J.F.; Prather, K.A.; Cohen, S.M. Tandem Postsynthetic Metal Ion and Ligand Exchange in Zeolitic Imidazolate Frameworks. Inorg. Chem. 2013, 52, 4011–4016. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.-B.B.; Liu, Q.; Trickett, C.A.; Gutiérrez-Puebla, E.; Monge, M.Á.; Cong, H.; Aldossary, A.; Deng, H.; Yaghi, O.M. Principles of Designing Extra-Large Pore Openings and Cages in Zeolitic Imidazolate Frameworks. J. Am. Chem. Soc. 2017, 139, 6448–6455. [Google Scholar] [CrossRef] [PubMed]
- Brekalo, I.; Kane, C.M.; Ley, A.N.; Ramirez, J.R.; Friščić, T.; Holman, K.T. Use of a “Shoe-Last” Solid-State Template in the Mechanochemical Synthesis of High-Porosity RHO-Zinc Imidazolate. J. Am. Chem. Soc. 2018, 140, 10104–10108. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Horike, S. Liquid, Glass and Amorphous Solid States of Coordination Polymers and Metal–Organic Frameworks. Nat. Rev. Mater. 2018, 3, 431–440. [Google Scholar] [CrossRef]
- Widmer, R.N.; Lampronti, G.I.; Chibani, S.; Wilson, C.W.; Anzellini, S.; Farsang, S.; Kleppe, A.K.; Casati, N.P.M.; Macleod, S.G.; Redfern, S.A.T.; et al. Rich Polymorphism of a Metal-Organic Framework in Pressure-Temperature Space. J. Am. Chem. Soc. 2019, 141, 9330–9337. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Geng, S.; Zhuo, M.; Chen, Y.; Zaworotko, M.J.; Cheng, P.; Zhang, Z. The Utility of the Template Effect in Metal-Organic Frameworks. Coord. Chem. Rev. 2019, 391, 44–68. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, S.; Wang, J. Understanding the Hydrogen Bonds in Ionic Liquids and Their Roles in Properties and Reactions. Chem. Commun. 2016, 52, 6744–6764. [Google Scholar] [CrossRef]
- Higgins, E.M.; Sherwood, J.A.; Lindsay, A.G.; Armstrong, J.; Massey, R.S.; Alder, R.W.; O’Donoghue, A.C. PKas of the Conjugate Acids of N-Heterocyclic Carbenes in Water. Chem. Commun. 2011, 47, 1559–1561. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Lalonde, M.B.; Bury, W.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T. Opening ZIF-8: A Catalytically Active Zeolitic Imidazolate Framework of Sodalite Topology with Unsubstituted Linkers. J. Am. Chem. Soc. 2012, 134, 18790–18796. [Google Scholar] [CrossRef]
- Morabito, J.V.; Chou, L.Y.; Li, Z.; Manna, C.M.; Petroff, C.A.; Kyada, R.J.; Palomba, J.M.; Byers, J.A.; Tsung, C.K. Molecular Encapsulation beyond the Aperture Size Limit through Dissociative Linker Exchange in Metal-Organic Framework Crystals. J. Am. Chem. Soc. 2014, 136, 12540–12543. [Google Scholar] [CrossRef]
- Ye, J.W.; Zhou, H.L.; Liu, S.Y.; Cheng, X.N.; Lin, R.B.; Qi, X.L.; Zhang, J.P.; Chen, X.M. Encapsulating Pyrene in a Metal-Organic Zeolite for Optical Sensing of Molecular Oxygen. Chem. Mater. 2015, 27, 8255–8260. [Google Scholar] [CrossRef]
- Cram, D.J.; Karbach, S.; Kim, H.E.; Knobler, C.B.; Maverick, E.F.; Ericson, J.L.; Helgeson, R.C. Host-Guest Complexation. 46. Cavitands as Open Molecular Vessels Form Solvates. J. Am. Chem. Soc. 1988, 110, 2229–2237. [Google Scholar] [CrossRef]
- Mottillo, C.; Friščić, T. Advances in Solid-State Transformations of Coordination Bonds: From the Ball Mill to the Aging Chamber. Molecules 2017, 22, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friščić, T.; Halasz, I.; Štrukil, V.; Eckert-Maksić, M.; Dinnebier, R.E. Clean and Efficient Synthesis Using Mechanochemistry: Coordination Polymers, Metal-Organic Frameworks and Metallodrugs. Croat. Chem. Acta 2012, 85, 367–378. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, J.; Zhang, P.; Dai, S. Mechanochemical Synthesis of Metal–Organic Frameworks. Polyhedron 2019, 162, 59–64. [Google Scholar] [CrossRef]
- Užarević, K.; Wang, T.C.; Moon, S.Y.; Fidelli, A.M.; Hupp, J.T.; Farha, O.K.; Friščić, T. Mechanochemical and Solvent-Free Assembly of Zirconium-Based Metal-Organic Frameworks. Chem. Commun. 2016, 52, 2133–2136. [Google Scholar] [CrossRef] [Green Version]
- Julien, P.A.; Užarević, K.; Katsenis, A.D.; Kimber, S.A.J.; Wang, T.; Farha, O.; Zhang, Y.; Casaban, J.; Germann, L.S.; Etter, M.; et al. In Situ Monitoring and Mechanism of the Mechanochemical Formation of a Microporous MOF-74 Framework. J. Am. Chem. Soc. 2016, 138, 2929–2932. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Frišcic, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for New and Cleaner Synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [Green Version]
- Beldon, P.J.; Fábián, L.; Stein, R.S.; Thirumurugan, A.; Cheetham, A.K.; Friščić, T. Rapid Room-Temperature Synthesis of Zeolitic Imidazolate Frameworks Using Mechanochemistry. Angew. Chem. Int. Ed. 2010, 49, 9640–9643. [Google Scholar] [CrossRef]
- Katsenis, A.D.; Puškarić, A.; Štrukil, V.; Mottillo, C.; Julien, P.A.; Užarević, K.; Pham, M.-H.H.; Do, T.-O.O.; Kimber, S.A.J.J.; Lazić, P.; et al. In Situ X-Ray Diffraction Monitoring of a Mechanochemical Reaction Reveals a Unique Topology Metal-Organic Framework. Nat. Commun. 2015, 6, 6662. [Google Scholar] [CrossRef] [Green Version]
- Mottillo, C.; Lu, Y.; Pham, M.H.; Cliffe, M.J.; Do, T.O.; Friščić, T. Mineral Neogenesis as an Inspiration for Mild, Solvent-Free Synthesis of Bulk Microporous Metal-Organic Frameworks from Metal (Zn, Co) Oxides. Green Chem. 2013, 15, 2121–2131. [Google Scholar] [CrossRef]
- Cliffe, M.J.; Mottillo, C.; Stein, R.S.; Bučar, D.K.; Friščić, T. Accelerated Aging: A Low Energy, Solvent-Free Alternative to Solvothermal and Mechanochemical Synthesis of Metal-Organic Materials. Chem. Sci. 2012, 3, 2495–2500. [Google Scholar] [CrossRef]
- Tanaka, S.; Kida, K.; Nagaoka, T.; Ota, T.; Miyake, Y. Mechanochemical Dry Conversion of Zinc Oxide to Zeolitic Imidazolate Framework. Chem. Commun. 2013, 49, 7884–7886. [Google Scholar] [CrossRef] [PubMed]
- Brekalo, I.; Yuan, W.; Mottillo, C.; Lu, Y.; Zhang, Y.; Casaban, J.; Holman, K.T.; James, S.L.; Duarte, F.; Williams, P.A.; et al. Manometric Real-Time Studies of the Mechanochemical Synthesis of Zeolitic Imidazolate Frameworks. Chem. Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Khay, I.; Chaplais, G.; Nouali, H.; Ortiz, G.; Marichal, C.; Patarin, J. Assessment of the Energetic Performances of Various ZIFs with SOD or RHO Topology Using High Pressure Water Intrusion-Extrusion Experiments. Dalt. Trans. 2016, 45, 4392–4400. [Google Scholar] [CrossRef]
- Houndonougbo, Y.; Signer, C.; He, N.; Morris, W.; Furukawa, H.; Ray, K.G.; Olmsted, D.L.; Asta, M.; Laird, B.B.; Yaghi, O.M. A Combined Experimental–Computational Investigation of Methane Adsorption and Selectivity in a Series of Isoreticular Zeolitic Imidazolate Frameworks. J. Phys. Chem. C 2013, 117, 10326–10335. [Google Scholar] [CrossRef]
- Forman, E.M.; Baniani, A.; Fan, L.; Ziegler, K.J.; Zhou, E.; Zhang, F.; Lively, R.P.; Vasenkov, S. Relationship between Ethane and Ethylene Diffusion inside ZIF-11 Crystals Confined in Polymers to Form Mixed-Matrix Membranes. J. Memb. Sci. 2020, 593, 117440. [Google Scholar] [CrossRef]
- Taylor, M.K.; Runčevski, T.; Oktawiec, J.; Gonzalez, M.I.; Siegelman, R.L.; Mason, J.A.; Ye, J.; Brown, C.M.; Long, J.R. Tuning the Adsorption-Induced Phase Change in the Flexible Metal-Organic Framework Co(Bdp). J. Am. Chem. Soc. 2016, 138, 15019–15026. [Google Scholar] [CrossRef]
- Yang, Q.-F.; Cui, X.-B.; Yu, J.-H.; Lu, J.; Yu, X.-Y.; Zhang, X.; Xu, J.-Q.; Hou, Q.; Wang, T.-G. A Series of Metal–Organic Complexes Constructed from in Situ Generated Organic Amines. CrystEngComm 2008, 10, 1534. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Masa, J.; Morales, D.M.; Tomanec, O.; Ranc, V.; Petr, M.; Wilde, P.; Chen, Y.T.; Zboril, R.; Schuhmann, W.; et al. Ultrathin 2D Cobalt Zeolite-Imidazole Framework Nanosheets for Electrocatalytic Oxygen Evolution. Adv. Sci. 2018, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Yao, J.; Liu, Q.; Zhong, Z.; Wang, H. Toluene-Assisted Synthesis of RHO-Type Zeolitic Imidazolate Frameworks: Synthesis and Formation Mechanism of ZIF-11 and ZIF-12. Dalt. Trans. 2013, 42, 16608–16613. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Díaz, A.; Villarroel-Rocha, J.; Ting, V.P.; Bimbo, N.; Sapag, K.; Mays, T.J. Flexible ZIFs: Probing Guest-Induced Flexibility with CO2, N2 and Ar Adsorption. J. Chem. Technol. Biotechnol. 2019, 94, 3787–3792. [Google Scholar] [CrossRef] [Green Version]
- Germann, L.S.; Katsenis, A.D.; Huskić, I.; Julien, P.A.; Užarević, K.; Etter, M.; Farha, O.K.; Friščić, T.; Dinnebier, R.E. Real-Time in Situ Monitoring of Particle and Structure Evolution in the Mechanochemical Synthesis of UiO-66 Metal–Organic Frameworks. Cryst. Growth Des. 2020, 20, 49–54. [Google Scholar] [CrossRef]
- Wu, T.; Bu, X.; Liu, R.; Lin, Z.; Zhang, J.; Feng, P. A New Zeolitic Topology with Sixteen-Membered Ring and Multidimensional Large Pore Channels. Chem. Eur. J. 2008, 14, 7771–7773. [Google Scholar] [CrossRef] [PubMed]
- Naumann, C.; Román, E.; Peinador, C.; Ren, T.; Patrick, B.O.; Kaifer, A.E.; Sherman, J.C. Expanding Cavitand Chemistry: The Preparation and Characterization of [n]Cavitands with n ≥ 4. Chem. Eur. J. 2001, 7, 1637–1645. [Google Scholar] [CrossRef]
- Kane, C.M.; Ugono, O.; Barbour, L.J.; Holman, K.T. Many Simple Molecular Cavitands Are Intrinsically Porous (Zero-Dimensional Pore) Materials. Chem. Mater. 2015, 27, 7337–7354. [Google Scholar] [CrossRef]
- Cram, D.J.; Stewart, K.D.; Goldberg, I.; Trueblood, K.N. Host-Guest Complexation. 49. Cavitands Containing Two Binding Cavities. J. Am. Chem. Soc. 1989, 111, 10. [Google Scholar]
- Kane, C.M.; Banisafar, A.; Dougherty, T.P.; Barbour, L.J.; Holman, K.T. Enclathration and Confinement of Small Gases by the Intrinsically 0D Porous Molecular Solid, Me,H,SiMe2. J. Am. Chem. Soc. 2016, 138, 4377–4392. [Google Scholar] [CrossRef]
- Kane, C.M. Crystalline Organic Cavitands As Microcavity Materials. Ph.D. Thesis, Georgetown University, Washington, DC, USA, August 2015. [Google Scholar]
- Bryant, J.A.; Blanda, M.T.; Vincenti, M.; Cram, D.J.; Donegani, G.; Fauser, V.G. Guest Capture during Shell Closure. J. Am. Chem. Soc. 1991, 113, 2167–2172. [Google Scholar] [CrossRef]
- Kaabel, S.; Stein, R.S.; Fomitšenko, M.; Järving, I.; Friščić, T.; Aav, R. Size-Control by Anion Templating in Mechanochemical Synthesis of Hemicucurbiturils in the Solid State. Angew. Chem. Int. Ed. 2019, 58, 6230–6234. [Google Scholar] [CrossRef]
Sample Availability: Limited quantities of the prepared compounds may be available upon request. |






| R | R’ | Y | n | Stereochemistry | Topological Result |
|---|---|---|---|---|---|
| H | Ph | CH2 | 4 | rccc | RHO |
| H | 4-MePh | CH2 | 4 | rccc | cag |
| Me | H | CH2 | 4 | rccc | RHO |
| Me | H | CH2 | 6 | rccc | RHO |
| Me | H | SiMe2 | 4 | rccc | cag |
| Me | i-Bu | CH2 | 4 | rccc | RHO |
| Me | Ph | CH2 | 4 | 87% rctt: 13% rccc | nog + RHO |
| Br | n-C5H11 | CH2 | 4 | rccc | RHO |
| Br | Ph | CH2 | 4 | rccc | RHO |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brekalo, I.; Deliz, D.E.; Kane, C.M.; Friščić, T.; Holman, K.T. Exploring the Scope of Macrocyclic “Shoe-last” Templates in the Mechanochemical Synthesis of RHO Topology Zeolitic Imidazolate Frameworks (ZIFs). Molecules 2020, 25, 633. https://doi.org/10.3390/molecules25030633
Brekalo I, Deliz DE, Kane CM, Friščić T, Holman KT. Exploring the Scope of Macrocyclic “Shoe-last” Templates in the Mechanochemical Synthesis of RHO Topology Zeolitic Imidazolate Frameworks (ZIFs). Molecules. 2020; 25(3):633. https://doi.org/10.3390/molecules25030633
Chicago/Turabian StyleBrekalo, Ivana, David E. Deliz, Christopher M. Kane, Tomislav Friščić, and K. Travis Holman. 2020. "Exploring the Scope of Macrocyclic “Shoe-last” Templates in the Mechanochemical Synthesis of RHO Topology Zeolitic Imidazolate Frameworks (ZIFs)" Molecules 25, no. 3: 633. https://doi.org/10.3390/molecules25030633