2.1. Structural Elucidation
The chemical structures of
8 and
9 were independently elucidated using HRESIMS and 1D and 2D NMR analyses (
Figures S1–S8 and
Table 1 and
Table 2). Their β-methoxyaspargine moieties were determined to be anti-relative configuration based on their large
3JHH coupling constant between H-2′′′ and H-3′′′. Additionally, the Cotton effects of
8 and
9 [[θ]
25(nm)(MeOH): −703 (252), 508 (310)] and [[
θ]
25(nm)(MeOH): −746 (252), 488 (309), respectively], were almost the same as those of
11 [[
θ]
25(nm)(MeOH): −5344 (251), 3999 (305)] [
4] in their circular dichroism (CD) spectra (
Figure S9). Thus,
8 and
9 were identified as cystobactamids 891-2 and 905-2 [
7], respectively. The complete assignments of the
13C-NMR data of
8 and
9 are reported for the first time in this study.
Compound
1 was not separated from
13 in SiO2 TLC but separated by ODS HPLC (
Figure S10). Compound
1 showed the same molecular formula (C
46H
44N
6O
15) as that of
13 based on their HRESIMS data in combination with their
1H and
13C NMR data (
Figures S11–S15 and
Table 1 and
Table 2). Various spectroscopic techniques, including 1D and 2D NMR experiments, were used for the structure elucidation of
1. The
1H-NMR and HMQC spectra of
1 were very similar to those of
13. The difference between the spectra of
1 and
13 is that the chemical shifts and coupling constants of H-2′′′ and H-3′′′ of the β-methoxyaspartic acid unit of
1 are more similar to those of
11 rather than
13 (
Table 3), suggesting that
1 could be a stereoisomer of
13 at the β-methoxyaspartic acid moiety. The signals at δ
H 4.75 (1H, dt,
J = 6.0, 12.1 Hz; δ
C 72.1), 4.31 (1H, dt,
J = 6.1, 12.2 Hz; δ
C 76.1), 1.37 (6H, d,
J = 6.0 Hz; δ
C 22.0), and 1.26 (6H, d,
J = 6.1 Hz; δ
C 22.3) suggested the presence of two isopropyl groups. Additionally, the
1H-NMR, COSY, and HMQC spectra of
1 indicated the presence of two
para-aminobenzoic acid units, a
para-nitrobenzoic acid unit, a 4-amino-3-isopropoxybenzoic acid unit, a 4-amino-2-hydroxyl-3-isopropoxybenzoic acid unit, and a β-methoxyaspartic acid unit. The NH
2 signal of the β-methoxyasparagine unit of
11 was not detected in the spectrum of
1 acquired in DMSO-
d6 (
Table 1), which, together with the molecular formula, suggested the presence of a β-methoxyaspartic acid fragment. The connectivities of these units were confirmed by the HMBC spectrum (
Figure 2). Thus, the planar structure of
1 was elucidated to be the same as that of
13 [
4].
The relative configuration at the β-methoxyaspartic acid unit of
1 was determined from
3JHH and
2JCH coupling constants and NOESY and NOE differential spectra (
Table 2 and
Figures S16–S18). The large coupling constant (8.1 Hz) between H-2′′′ and H-3′′′ indicated the anti-
configuration. The
2JCH coupling constants were measured in DMSO-
d6 by a HECADE experiment (
Figure S16). The large coupling constant (5.11 Hz) between H-2′′′ and C-3′′′ indicated that H-2′′′ and OMe are in a
gauche. Together with these coupling constants, the NOEs between H-3′′′ and NH-6′′′, from NH-8′′ to H-3′′′ and OMe, and from H-2′′′ to OMe confirmed that
1 had the same relative configuration as
11 (
Figure S19). An unexpected NOE from H-2′′′ to H-3′′′ suggested the presence of the other rotamer as a minor conformer as detected previously in
13 [
4]. The Cotton effect [[
θ]
25(nm)(MeOH): −415 (250), 345 (304)] in the CD spectra of
1 was almost the same as that [[
θ]
25(nm)(MeOH): −5344 (251), 3999 (305)] of
11 [
4] (
Figure S9). These data clearly indicated that
1 had the same configuration (
S*R*) as
11. Thus,
1 was elucidated to be a stereoisomer of
13.
The HRESIMS data of
2 gave a protonated molecule at
m/z 890.3415 [M + H]
+ (calcd. 890.3362 for C
46H
48N
7O
12), which in combination with
1H and
13C NMR spectra suggested a molecular formula of C
46H
47N
7O
12 (
Figures S20–S24 and
Table 1 and
Table 2). The
1H NMR spectrum of
2 was similar to that of
11. The difference was the appearance of the aromatic proton signals [δ
H 7.75 (2H, d,
J = 8.8 Hz; δ
C 129.9) and 6.62 (2H, d,
J = 8.6 Hz; δ
C 112.9)] for an 1,4-disubstituted phenyl moiety instead of signals (δ
H 8.39 and 8.21) for the
para-nitrobenzoic acid unit in
11 (
Table 1). In the HMBC spectrum (
Figure 2), the proton at δ
H 6.62 showed long-range coupling to the carbons at δ
C 113.1 and 120.9, and the proton at δ
H 7.75 showed long-range coupling to the carbons at δ
C 165.9, 152.8, and 129.9. These
13C NMR data were consistent with those of
para-aminobenzoic acid [
8]. Together with the molecular formula, these spectroscopic data indicated the presence of a
para-aminobenzoic acid unit instead of a
para-nitrobenzoic acid unit. The connectivity of the remaining units was confirmed by the HMBC spectrum (
Figure 2). Thus, the planar structure of
2 was identified as that of a new, C-5′′′′′ amino derivative of
11.
The relative configuration of the β-methoxyasparagine moiety of
2 was determined from
3JHH and
2JCH coupling constants and NOESY and NOE differential spectra (
Table 1 and
Figures S25–S27). The coupling constant between H-2′′′ and H-3′′′ was 8.0 Hz, indicating the
anti configuration. Similar to
1, the large
2JCH coupling constant (5.49 Hz) between H-2′′′ and C-3′′′ indicated a
gauche conformation of H-2′′′ and OMe. The NOEs between H-3′′′ and NH-6′′′, from NH-8′′ to H-3′′′ and OMe, and from H-2′′′ to OMe supported the same relative stereochemistry as
11. The Cotton effect [[
θ]
25(nm)(MeOH): −1278 (256), 1301 (313)] of
2 was almost the same as that [[
θ]
25(nm)(MeOH): −5344 (251), 3999 (305)] of
11 in CD spectra (
Figure S9). Thus,
2 was determined to be a new, C-5′′′′′ amino derivative of
11.
The molecular formula of
3 was determined to be C
39H
24N
6O
11 based on its HRESIMS data in combination with its
1H and
13C NMR data (
Figures S28–S30 and
Table 1 and
Table 2). The
1H NMR spectrum of
3 was similar to that of
2. The difference between the spectra of
3 and
2 was that the overlapping aromatic proton signals [δ
H 7.84 (4H, brs)] and the amino signal [δ
H 10.02 (1H, s)] of the
para-aminobenzoic acid unit in the spectrum of
2 were not observed in the spectrum of
3. Additionally, detailed analysis of its
1H NMR,
13C NMR, COSY, and HMQC spectra suggested the presence of two
para-aminobenzoic acid fragments, a 4-amino-3-isopropoxybenzoic acid fragment, a 4-amino-2-hydroxyl 3-isopropoxybenzoic acid fragment, and a β-methoxyasparagine fragment. These spectroscopic data suggested that
3 had one fewer
para-aminobenzoic acid unit than were present in
2. The aromatic proton at δ
H 7.58 (H-3′′′′ and H-7′′′′) of one
para-aminobenzoic acid unit [δ
H 7.58 (2H, overlapped; δ
C 129.0) and 6.57 (2H, d,
J = 8.1 Hz; δ
C 112.7)] and the amine proton at δ
H 8.00 (6′′′-NH) have HMBC correlations with the carbonyl carbon at δ
C 166.0 (C-1′′′′), which in turn showed long-range coupling with the α-proton at 4.82 (H-2′′′) of the β-methoxyasparagine unit (
Figure 2). These HMBC data indicated that the terminal
para-aminobenzoic acid unit of
2 was absent in
3. The remaining structural fragments were confirmed by the HMBC spectrum (
Figure 2). Thus, the planar structure of
3 was identified as that of a new derivative of
2 without the terminal
para-aminobenzoic acid unit.
The relative configuration of the β-methoxyasparagine moiety of
3 was determined based on
3JHH and
2JCH coupling constants and NOESY and NOE differential spectra (
Table 1 and
Figures S34–S36). H-2′′′ and H-3′′′ were
anti to each other based on their large
3JHH coupling constant (8.0 Hz). On the basis of the large
2JCH coupling constant (6.09 Hz) between H-2′′′ and C-3′′, H-2′′′ and OMe are in a
gauche. The NOEs between H-3′′′ and NH-6′′′, from NH-8′′ to H-3′′′ and OMe, and from H-2′′′ to OMe supported the same relative stereochemistry as
2. The Cotton effects [[
θ]
25(nm)(MeOH): −707 (260), 976 (301)] of
3 were almost the same as those [[
θ]
25(nm)(MeOH): −1278 (256), 1301 (313)] of
2 (
Figure S9). Thus,
3 was determined to be a new derivative of
2 without the terminal
para-aminobenzoic acid unit.
Compound
10 was identified as cystobactamid 507 [
5] based on its ESIMS data in combination with its
1H and
13C NMR data (
Figures S37–S40 and
Table 4 and
Table 5). The molecular formula of
4 was determined to be C
25H
25O
7N
3 based on its HRESIMS data in combination with its
1H and
13C NMR spectra (
Figures S41–S44 and
Table 4 and
Table 5). The
1H NMR data acquired in CD
3OD revealed the presence of nine aromatic protons, two oxygenated methylenes at δ
H 4.29 (2H, q,
J = 7.0 Hz) and 4.16 (2H, q,
J = 7.0 Hz), and two methyl groups at δ
H 1.58 (3H, t,
J = 7.0 Hz) and 1.45 (3H, t,
J = 7.0 Hz) (
Table 4). The
1H-NMR and HMQC spectra of
4 indicated the presence of 1,4-disubstituted benzene, 1,2,4-trisubstituted benzene, 1,2,3,4-tetrasubstituted benzene, and two ethoxy groups. This result suggested the presence of two ethoxy groups in
4 instead of the two isopropoxyl groups seen in
10. This was confirmed by the HMBC correlations from the methylene protons at δ
H 4.16 of one ethoxy group to the carbon at δ
C 137.9 of the 1,2,4-trisubstituted benzene and from the methylene protons at δ
H 4.02 of the other ethoxy group to the carbon at δ
C 148.0 of the 1,2,4-trisubstituted benzene (
Figure 3). Thus,
4 was determined to be a new, C-4 and C-4′ diethoxylated derivative at of
10.
The molecular formula of
5 was determined to be C
25H
25O
7N
3 based on its HRESIMS data in combination with its
1H and
13C NMR data (
Figures S45–S48 and
Table 4 and
Table 5). The
1H NMR and HMQC data of
5 were similar to those of
10. The differences were that a signal for a methoxy group at δ
H 3.97 was observed instead of signals of an isopropoxy group. The location of the methoxy group was determined based on the HMBC correlation from the methoxy proton signal to the carbon at δ
C 149.4 of the 1,2,4-trisubstituted benzene (
Figure 3). Thus,
5 was determined to be a new, C-4 methoxylated derivative of
10.
The molecular formula of
6 was determined to be C
25H
25O
7N
3 based on its HRESIMS data in combination with
1H and
13C NMR data (
Figures S49–S52 and
Table 4 and
Table 5). The NMR spectra of
6 were similar to those of
10 except for the presence of ethoxy signals [δ
H 4.02 (1H, q,
J = 7.0 Hz; δ
C 68.9) and 1.34 (3H, t,
J = 7.0 Hz; δ
C 15.6)] instead signals for an isopropyl fragment. In the HMBC spectrum, the methylene protons at δ
H 4.02 of the ethoxy group showed long-range coupling to the carbon at δ
C 138.6, which in turn was correlated with the amine proton at δ
H 9.14 and the aromatic proton at δ
H 7.68 (
Figure 3). These correlations indicated the linkage of the ethoxy group to C-3 of the 1,2,3,4-tetrasubstituted benzene. The HMBC spectrum confirmed the remaining structural features of
6. Thus,
6 was elucidated as a new, C-4′ ethoxylated derivative at of
10.
The molecular formula of
7 was determined to be C
25H
25O
7N
3 based on its HRESIMS data in combination with its
1H and
13C NMR data (
Figures S53–S58 and
Table 4 and
Table 5). The NMR spectra of
7 were similar to those of
6. The major difference in the
1H NMR and HMQC data were that the methine proton of the isopropyl group of
6 was downfield-shifted from δ
H 4.77 to 4.36 in the spectra of
7. This result suggested that the isopropyl group might be connected to the 1,2,3,4-tetrasubstituted benzene as it is in
5. HMBC correlations were observed from the methine proton of the isopropyl group to the carbon at δ
C 138.1 (C-4′) of the 1,2,3,4-tetrasubstituted benzene and from the methylene proton of the ethoxy group to the carbon at δ
C 148.3 (C-4) of 1,2,4-trisubstituted benzene (
Figure 3). The HMBC spectrum confirmed the remaining structural features of
7. Thus,
7 was determined to be as a new, C-4 ethoxylated derivative of
10.
2.2. Antibacterial and E. Coli DNA Gyrase-Inhibitory Activities of the New Compounds
The antibacterial activities of
1–
10 against clinically important Gram-positive and Gram-negative pathogens were evaluated compared with those of
11–
13 and ciprofloxacin (
Table 6 and
Table S1). Additionally, their effects on the supercoiling activity of
E. coli DNA gyrase were investigated using ciprofloxacin and nalidixic acid, as positive controls, (
Figure S59 and
Table 7) because the main target of
11 was reported to be DNA gyrase [
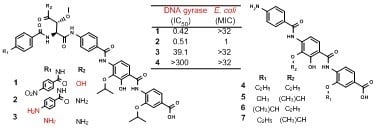
5]. Compound
1, the β-methoxyaspartic acid derivative of
11, showed weaker antibacterial activity against Gram-positive bacteria (MICs of 8–4 μg/mL) compared with that of
11, and it exhibited no antibacterial activity against both Gram-negative bacteria at 32 μg/mL. However,
1 showed stronger
E. coli DNA gyrase inhibition (IC
50 of 0.42 μM) than was observed in
11 (0.95 μM). Considering that
13, the stereoisomer of
1, retained antibacterial activity against
E. coli, these results suggested that the β-methoxyasparagine moiety and its stereochemistry in
11 influences cellular uptake in both Gram-positive and Gram-negative bacteria. The stronger antibacterial activity against
E. coli of
12 could be due to its stronger
E. coli DNA gyrase inhibition than
11. Compound
2,
8, and
9 showed antibacterial activities similar to that of
11 against both Gram-positive and Gram-negative bacteria except against
P. aeruginosa and
K. pneumonia. Compound
2, with an amino moiety instead of the nitro moiety on the
para-nitrobenzoic acid unit (relative to
11), did not inhibit the growth of
P. aeruginosa or
K. pneumonia at 32 μg/mL, while
8 and
9, the derivatives in which the isopropyl chain at C-4 of
11 has been shortened, showed weak antibacterial activities against
P. aeruginosa but did not inhibit the growth of
K. pneumonia at 32 μg/mL. However,
2,
8, and
9 showed strong
E. coli DNA gyrase inhibition with IC
50 values of 0.05–0.51 μM, making them more potent than
11. Considering that
11 was reported to inhibit DNA gyrases of both
E. coli and
P. aeruginosa with similar potencies [
9], these results suggested that the nitro group and the isopropyl chain could be important for permeability into
P. aeruginosa and
K. pneumonia. Another explanation of the discrepancy between
E. coli DNA gyrase inhibition and antibacterial activity of these respective compounds might also be due to an improved efflux.
On the other hand,
3, a derivative of
11 with the
para-nitrobenzoic acid unit removed, exhibited no antibacterial activities against any of the tested bacterial strains at 32 μg/mL, which was consistent with its dramatically lower ability to inhibit DNA gyrase (IC
50 of 39.1 μM). These results suggested that the
para-aminobenzoic acid unit is critical to the inhibition of DNA gyrase and bacterial growth. Compounds
4–
7 and
10, derivatives missing the
para-aminobenzoic acid unit and β-methoxyasparagine moiety, exhibited no antibacterial activities against any of the tested bacterial strains at 32 μg/mL and showed no DNA gyrase inhibition even at 300 μM. Although
10 was reported to show weak antibacterial and DNA gyrase-inhibitory activities against Gram-negative and Gram-positive bacteria (MICs of 4–65 μg/mL and IC
50 of 20.2 μM, respectively) [
5],
10 and its derivatives
4–
7 did not display those activities in this study.