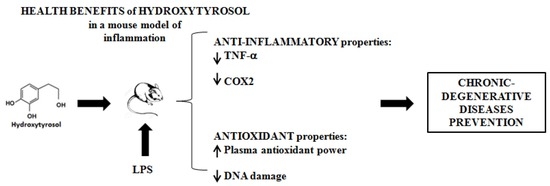
Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation
Abstract
:1. Introduction
2. Results
2.1. HT Inhibits the COX2 Gene Expression in LPS-Stimulated Mouse Model
2.2. HT Reduces the TNF-α Cytokine Secretion in LPS Stimulated Mouse Model
2.3. HT Improves the Antioxidant Power of Plasma in LPS Stimulated Mouse Model
2.4. HT Prevents the DNA Damage Induced by LPS-Stimulation in Mouse Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Animals Sacrifice
4.4. COX2 mRNA Analysis by Real-Time PCR
4.5. TNF-α Cytokine Determination
4.6. DNA Damage Assessment
4.7. Comet Detection
4.8. Antioxidant Plasma Power Quantification
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| COX2 | cyclooxygenase-2 |
| HT | hydroxytyrosol |
| EVOO | extra-virgin olive oil |
| FRAP | ferric reducing anti-oxidant power |
| i.p. | intraperitoneal injection |
| NSAIDs | non steroideal anti-inflammatory drugs |
| PBMC | peripheral blood mononuclear cells |
| qPCR | quantitative PCR |
| ROS | reactive oxygen species |
| TNF-α | tumor necrosis factor alfa |
References
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Wajan, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signalling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, F.; Ferroni, P.; Palmirotta, R.; Portarena, I.; Formica, V.; Roselli, M. TNF/VEGF Cross-talk in Chronic Inflammation-related Cancer Initiation and Progression: An Early Target in Anticancer Therapeutic Strategy. In Vivo 2007, 21, 147–162. [Google Scholar] [PubMed]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamy, S.; Ben Saad, A.; Zgheib, A.; Annabi, B. Olive oil compounds inhibit the paracrine regulation of TNF-α-induced endothelial cell migration through reduced glioblastoma cell cyclooxygenase-2 expression. J. Nutr. Biochem. 2016, 27, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit. Rev. Clin. Lab. Sci. 2009, 46, 241–281. [Google Scholar] [CrossRef] [PubMed]
- Milic, M.; Frustaci, A.; Del Bufalo, A.; Sánchez-Alarcón, J.; Valencia-Quintana, R.; Russo, P.; Bonassi, S. DNA damage in non-communicable diseases: A clinical and epidemiological perspective. Mutat. Res. 2015, 776, 118–127. [Google Scholar] [CrossRef]
- Magrone, T.; Perez de Heredia, F.; Jirillo, E.; Morabito, G.; Marcos, A.; Serafini, M. Functional foods and nutraceuticals as therapeutic tools for the treatment of diet-related diseases. Can. J. Physiol. Pharmacol. 2013, 91, 387–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, R.; Libuy, M.; Feliu, F.; Hasson, D. Polyphenols in disease: from diet to supplements. Curr. Pharm. Biotechnol. 2014, 15, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Capurso, C.; Capurso, A.; Massaro, M. Vascular effects of the Mediterranean diet-part II: Role of omega-3 fatty acids and olive oil polyphenols. Vascul. Pharmacol. 2014, 6, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCa and PKCb1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.; López-Miranda, J.; Pérez-Martínez, P.; Jiménez, Y.; Marín, C.; Gómez, P.; Fernández, J.M.; Caballero, J.; Delgado-Lista, J.; Pérez-Jiménez, F. Chronic effects of a high-fat diet enriched with virgin olive oil and a low-fat diet enriched with alpha-linolenic acid on postprandial endothelial function in healthy men. Br. J. Nutr. 2008, 100, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Fabiani, R.; Servili, M.; Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 2013, 24, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Fabiani, R.; Sepporta, M.V.; Rosignoli, P. The hydroxytyrosol-dependent increase of TNF-α in LPS-activated human monocytes is mediated by PGE2 and adenylate cyclase activation. Toxicol. In Vitro 2015, 29, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Page, T.H.; Turner, J.J.O.; Brown, A.C.; Timms, E.M.; Inglis, J.J.; Brennan, F.M.; Foxwell, B.M.J.; Ray, K.P.; Feldmann, M. Nonsteroidal anti-inflammatory drugs increase TNF production in rheumatoid synovial membrane cultures and whole blood. J. Immunol. 2010, 185, 3694–3701. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases: Progress Monitor 2017; World Health Organization: Geneva, Switzerland, 2017; pp. 1–232. [Google Scholar]
- Tomé-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef] [PubMed]
- Copeland, S.; Shaw Warren, H.; Lowry, S.F.; Calvano, S.E.; Remick, D. Inflammation and the Host Response to Injury Investigators. Acute Inflammatory Response to Endotoxin in Mice and Humans. Clin. Diagn. Lab. Immunol. 2005, 12, 60–67. [Google Scholar] [PubMed]
- Ciou, S.Y.; Hsu, C.C.; Kuo, Y.H.; Chao, C.Y. Effect of wild bitter gourd treatment on inflammatory responses in BALB/c mice with sepsis. BioMedicine 2014, 4, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.J.; Lai, Y.R.; Chen, Y.L.; Chang, Y.H.; Li, Z.Y.; Huang, W.C. Matrine Attenuates COX-2 and ICAM-1 Expressions in Human Lung Epithelial Cells and Prevents Acute Lung Injury in LPS-Induced Mice. Mediators Inflamm. 2016, 2016, 3630485. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Hosono, K.; Tsuru, S.; Kurashige, C.; Sekiguchi, K.; Akira, S.; Uematsu, S.; Okamoto, H.; Majima, M. Roles of mPGES-1, an inducible prostaglandin E. synthase, in enhancement of LPS-induced lymphangiogenesis in a mouse peritonitis model. Life Sci. 2015, 142, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Expósito, M.J.; Martínez-Martos, J.M. Anti-Inflammatory and Antitumor Effects of Hydroxytyrosol but Not Oleuropein on Experimental Glioma In Vivo. A Putative Role for the Renin-Angiotensin System. Biomedicines 2018, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Crespo, M.C.; Iglesias-Gutierrez, E.; Martín, R.; Gil-Zamorano, J.; Tomas-Zapico, C.; Burgos-Ramos, E.; Correa, C.; Gómez-Coronado, D.; Lasunción, M.A.; et al. Hydroxytyrosol supplementation modulates the expression of miRNAs in rodents and in humans. J. Nutr. Biochem. 2016, 34, 146–155. [Google Scholar]
- Yang, X.; Jing, T.; Li, Y.; He, Y.; Zhang, W.; Wang, B.; Xiao, Y.; Wang, W.; Zhang, J.; Wei, J.; et al. Hydroxytyrosol Attenuates LPS-Induced Acute Lung Injury in Mice by Regulating Autophagy and Sirtuin Expression. Curr. Mol. Med. 2017, 17, 149–159. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, B.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fidalgo, S.; Sanchez de Ibarguen, L.; Cardeno, A.; Alarcon de la Lastra, C. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur. J. Nutr. 2012, 51, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; Alarcón de la Lastra, C. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J. Nutr. Biochem. 2013, 24, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fidalgo, S.; Villegasa, I.; Aparicio-Soto, M.; Cárdeno, A.; Rosillo, A.; González-Benjumea, A.; Marset, A.; López, O.; Maya, I.; Fernández-Bolaños, J.G.; et al. Effects of dietary virgin olive oil polyphenols: hydroxytyrosyl acetate and 3,4-dihydroxyphenylglycol on DSS-induced acute colitis in mice. J. Nutr. Biochem. 2015, 26, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.; Patrignani, P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J. Pain Res. 2015, 8, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Sultana, F.; Rasool, M. A novel therapeutic approach targeting rheumatoid arthritis by combined administration of morin, a dietary flavanol and non-steroidal anti-inflammatory drug indomethacin with reference to pro-inflammatory cytokines, inflammatory enzymes, RANKL and transcription factors. Chem. Biol. Interact. 2015, 230, 58–70. [Google Scholar] [PubMed]
- Masuelli, L.; Di Stefano, E.; Fantini, M.; Mattera, R.; Benvenuto, M.; Marzocchella, L.; Sacchetti, P.; Focaccetti, C.; Bernardini, R.; Tresoldi, I.; et al. Resveratrolpotentiates the in vitro and in vivo anti-tumoraleffects of curcumin in head and neckcarcinomas. Oncotarget 2014, 5, 10745–10762. [Google Scholar] [CrossRef]
- Wang, S.; Chen, R.; Zhong, Z.; Shi, Z.; Chen, M.; Wang, Y. Epigallocatechin-3-gallate potentiates the effect of curcumin in inducing growth inhibition and apoptosis of resistant breast cancer cells. Am. J. Chin. Med. 2014, 42, 1279–1300. [Google Scholar] [CrossRef]
- Aunon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxyty-rosol. Food Chem. Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef]
- Bitler, C.M.; Viale, T.M.; Damaj, B.; Crea, R. Hydrolyzed olive vegetation water in mice has anti-inflammatory activity. J. Nutr. 2005, 135, 1475–1479. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell. Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn-Schmied Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Lubberts, E.; Alarcon-de-la-Lastra, C. Preventive effects of dietary hydroxytyrosol acetate, an extra virgin olive oil polyphenol in murine collagen-induced arthritis. Mol. Nutr. Food Res. 2015, 59, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, M.; Sarria, B.; Largo, C.; Martınez-Lopez, S.; Madrona, A.; Espartero, J.L.; Bravo, L.; Mateos, R. Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct. 2014, 5, 1556–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenzuela, R.; Illesca, P.; Echeverría, F.; Espinosa, A.; Rincón-Cervera, M.Á.; Ortiz, M.; Hernandez-Rodas, M.C.; Valenzuela, A.; Videla, L.A. Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-α and Nrf2 activation, and NF-κB down-regulation. Food Funct. 2017, 8, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jiang, K.; Zhang, T.; Zhao, G.; Deng, G. Hydroxytyrosol exerts an anti-inflammatory effect by suppressing Toll-like receptor 2 and TLR 2 downstream pathways in Staphylococcus aureus-induced mastitis in mice. J. Funct. Foods 2017, 35, 595–604. [Google Scholar] [CrossRef]
- Carito, V.; Ciafrè, S.; Tarani, L.; Ceccanti, M.; Natella, F.; Iannitelli, A.; Tirassa, P.; Chaldakov, G.N.; Ceccanti, M.; Boccardo, C.; Fiore, M. TNF-α and IL-10 modulation induced by polyphenols extracted by olive pomace in a mouse model of paw inflammation. Ann. Ist. Super. Sanità 2015, 51, 382–386. [Google Scholar]
- Covas, M.I.; de la Torre, R.; Fitó, M. Virgin olive oil: A key food for cardiovascular protection. Br. J. Nutr. 2015, 113, 19–28. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Corella, D.; Castañer, O.; Lamuela-Raventos, R.M.; Salas-Salvadó, J.; Martínez-González, M.A.; Ros, E.; Estruch, R. Long-Term Immunomodulatory Effects of a Mediterranean Diet in Adults at High Risk of Cardiovascular Disease in the PREvención con DIetaMEDiterránea (PREDIMED) Randomized Controlled Trial. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef]
- Corsi, R.; Mosti, G.; Cavezzi, A.; Urso, S.U.; Dimitrova, G.; Fioroni, E.; Colucci, R.; Quinzi, V. A Polyphenol-Based Multicomponent Nutraceutical in Dysmetabolism and Oxidative Stress: Results from a Pilot Study. J. Diet. Suppl. 2018, 15, 1–8. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.S.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: a randomized controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Di Renzo, L.; Trombetta, D.; Smeriglio, A.; Bernardini, S.; Cioccoloni, G.; Costa de Miranda, R.; Gualtieri, P.; SinibaldiSalimei, P.; De Lorenzo, A. Antioxidant Effects of a Hydroxytyrosol-Based Pharmaceutical Formulation on Body Composition, Metabolic State, and Gene Expression: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Oxid. Med. Cell Longev. 2017, 2017, 2473495. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.C.; Tomé-Carneiroa, J.; Burgos-Ramosa, E.; Kohen, V.L.; Espinosa, M.I.; Herranza, J.; Visioli, F. One-week administration of hydroxytyrosol to humans does notactivate Phase II enzymes. Pharmacol. Res. 2015, 95, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Glukhov, I.L.; Sirota, N.P.; Kuznetsova, E.A. DNA damage in human mononuclear cells induced by bacterial endotoxin. Bull. Exp. Biol. Med. 2008, 46, 301–313. [Google Scholar] [CrossRef]
- Serebrovska, Z.; Swanson, R.J.; Portnichenko, V.; Shysh, A.; Pavlovich, S.; Tumanovska, L.; Dorovskych, A.; Lysenko, V.; Tertykh, V.; Bolbukh, Y.; et al. Anti-inflammatory and antioxidant effect of cerium dioxide nanoparticles immobilized on the surface of silica nanoparticles in rat experimental pneumonia. Biomed. Pharmacother. 2017, 92, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Tuo, J.; Loft, S.; Poulsen, H.E. Enhanced benzene-induced DNA damage in PMA-stimulated cells in vitro and in LPS-treated animals. Free Radic. Biol. Med. 1999, 26, 801–818. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Sepporta, M.V.; Fabiani, R. In vitro chemo-preventive activities of hydroxytyrosol: the main phenolic compound present in extra-virgin olive oil. Food Funct. 2016, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Servili, M.; Montedoro, G.F.; Morozzi, G. Oxidative DNA damage is prevented by extracts of olive oil, hydroxytyrosol, and other olive phenolic compounds in human blood mononuclear cells and HL60 cells. J. Nutr. 2008, 138, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Sepporta, M.V.; Rosignoli, P.; Morozzi, G.; Servili, M.; Fabiani, R. Preventive activity of olive oil phenolic compounds on alkene epoxides induced oxidative DNA damage on human peripheral blood mononuclear cells. Nutr. Cancer 2014, 66, 1322–1330. [Google Scholar] [CrossRef]
- Larrosa, M.; Azorín-Ortuño, M.; Yañez-Gascón, M.J.; García-Conesa, M.T.; Tomás-Barberán, F.; Espín, J.C. Lack of effect of oral administration of resveratrol in LPS-induced systemic inflammation. Eur. J. Nutr. 2011, 50, 673–680. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Kwak, H.K. Antioxidant effects of cranberry powder in lipopolysaccharide treated hypercholesterolemic rats. Prev. Nutr. Food Sci. 2014, 19, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Mani Senthil Kumar, K.T.; Gorain, B.; Roy, D.K.; ZothanpuiaSamanta, S.K.; Pal, M.; Biswas, P.; Roy, A.; Adhikari, D.; Karmakar, S.; Sen, T. Anti-inflammatory activity of Acanthus ilicifolius. J. Ethnopharmacol. 2008, 120, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Ma, A.G.; Duthie, S.J. The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat. Res. 1995, 336, 69–77. [Google Scholar] [CrossRef]
- Collins, A.R.; Duthie, S.J.; Dobson, V.L. Direct enzyme detection of endogenous oxidative base damage in human lymphocytes DNA. Carcinogenesis 1993, 14, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How Do Nutritional Antioxidants Really Work: Nucleophilic Tone and Para-Hormesis Versus Free Radical Scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation. Molecules 2018, 23, 3212. https://doi.org/10.3390/molecules23123212
Fuccelli R, Fabiani R, Rosignoli P. Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation. Molecules. 2018; 23(12):3212. https://doi.org/10.3390/molecules23123212
Chicago/Turabian StyleFuccelli, Raffaela, Roberto Fabiani, and Patrizia Rosignoli. 2018. "Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation" Molecules 23, no. 12: 3212. https://doi.org/10.3390/molecules23123212