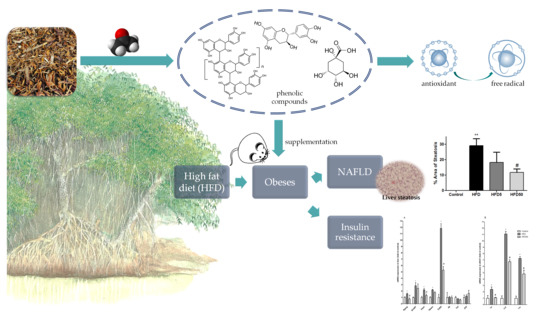
Modulatory Effect of Polyphenolic Compounds from the Mangrove Tree Rhizophora mangle L. on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in High-Fat Diet Obese Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Extracts
2.2. In Vitro Activities of the Extract
2.3. Hepatoprotective and Insulin Sensitization after AERM Administration In Vivo
3. Materials and Methods
3.1. Sample Taxon
3.2. Chemical Characterization
3.2.1. Preparation of Plant Extract
3.2.2. HPLC-PDA
3.2.3. FIA-ESI-IT-MS/MS
3.3. In Vitro Assays
3.3.1. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
3.3.2. Lipase Activity Assay
3.3.3. Alpha-Amylase Activity Assay
3.4. Experimental Model of Diet-Induced Obesity
3.4.1. Animals
3.4.2. Diet-Induced Obesity and R. mangle Treatment
3.4.3. Blood Glucose Levels and Insulin Tolerance Tests
3.4.4. Necropsy and Sample Collection
3.4.5. Hepatic Analyses
3.4.6. Serum Analyses
3.4.7. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| AERM | Acetonic extract of Rhizophora mangle barks |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FA | Formic acid |
| FFA | Free-fat-acid |
| HFD | High-fat diet |
| HPLC-PDA | High-performance liquid chromatography coupled to photodiode array |
| HRF | Heterocyclic ring fission |
| FIA-ESI-IT-MS | Flow injection analysis electrospray-ion trap mass spectrometry |
| kiTT | Insulin tolerance test |
| m/z | mass/charge ratio |
| NAFLD | Non-alcoholic fatty liver disease |
| Pas | Proanthocyanidins |
| PCR | Polymerase chain reaction |
| RDA | Retro Diels-Alder |
| ROS | Reactive oxygen species |
| SPE | Solid-phase extraction |
| T2D | Type 2 diabetes |
| TEAC | Trolox equivalent antioxidant activity |
| QM | Quinone-methide |
References
- Cordeiro, C.; Costa, T. Evaluation of solid residues removed from a mangrove swamp in the São Vicente Estuary, SP, Brazil. Mar. Pollut. Bull. 2010, 60, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Nebula, M.; Harisankar, H.; Chandramohanakumar, N. Metabolites and bioactivities of Rhizophoraceae mangroves. Nat. Prod. Bioprospect. 2013, 3, 207–232. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Mao, W.; Guo, X.; Wu, Y.; Li, C.; Lu, Z.; Su, G.; Li, X.; Liu, Z.; Guo, R. Ginkgo biloba extract for patients with early diabetic nephropathy: A systematic review. Evid. Based Complement. Altern. Med. 2013, 2013, 17. [Google Scholar] [CrossRef]
- Valdivia-Correa, B.; Gómez-Gutiérrez, C.; Uribe, M.; Méndez-Sánchez, N. Herbal medicine in Mexico: A cause of hepatotoxicity. A critical review. Int. J. Mol. Sci. 2016, 17, 235. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Aguilara, F.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.; Flores-Saenz, J. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; ISBN 92-4-156525-X. [Google Scholar]
- Associação Brasileira para Estudo da Obesidade e da Síndrome Metabólica (ABESO). Mapa da Obesidade; Brazilian Association for Obesity Study: São Paulo, Brazil, 2017. [Google Scholar]
- Zhang, Y.; Feng, F.; Chen, T.; Li, Z.; Shen, Q.W. Antidiabetic and antihyperlipidemic activities of Forsythia suspensa (Thunb.) Vahl (fruit) in streptozotocin-induced diabetes mice. J. Ethnopharmacol. 2016, 192, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2017, 8, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Azman, A.-S.; Othman, I.; Saraswati, S.V.; Chan, K.-G.; Lee, L.-H. Mangrove rare actinobacteria: Taxonomy, natural compound, and discovery of bioactivity. Front. Microbiol. 2015, 6, 856. [Google Scholar] [CrossRef] [PubMed]
- de Faria, F.M.; Luiz-Ferreira, A.; Socca, E.A.R.; de Almeida, A.C.A.; Dunder, R.J.; Manzo, L.P.; da Silva, M.A.; Vilegas, W.; Rozza, A.L.; Pellizzon, C.H. Effects of Rhizophora mangle on experimental colitis induced by TNBS in rats. Evid. Based Complement. Altern. Med. 2012, 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Antioxidant and potential anti-inflammatory activity of extracts and formulations of white tea, rose, and witch hazel on primary human dermal fibroblast cells. J. Inflamm. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Pan, Q.-H.; Shi, Y.; Duan, C.-Q. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules 2008, 13, 2674–2703. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, S.; Koboziev, I.; Park, O.-H.; Oldewage-Theron, W.; Shen, C.-L.; Moustaid-Moussa, N. Anti-inflammatory and anti-obesity properties of food bioactive components: Effects on adipose tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef] [PubMed]
- de-Faria, F.M.; Almeida, A.C.A.; Luiz-Ferreira, A.; Takayama, C.; Dunder, R.J.; da Silva, M.A.; Salvador, M.J.; Abdelnur, P.V.; Eberlin, M.N.; Vilegas, W. Antioxidant action of mangrove polyphenols against gastric damage induced by absolute ethanol and ischemia-reperfusion in the rat. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Rohr, G.E.; Meier, B.; Sticher, O. Evaluation of different detection modes for the analysis of procyanidins in leaves and flowers of Crataegus spp. Part I. Diode array and electrochemical detection. Phytochem. Anal. 2000, 11, 106–112. [Google Scholar] [CrossRef]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P.; Venkatesan, M.; Ramu, A. Mangrove plants as a source of lead compounds for the development of new antiplasmodial drugs from South East coast of India. Parasitol. Res. 2011, 108, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Oo, C.W.; Pizzi, A.; Pasch, H.; Kassim, M.J. Study on the structure of mangrove polyflavonoid tannins with MALDI-TOF mass spectrometry. J. Appl. Polym. Sci. 2008, 109, 963–967. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Lin, Y.-M.; Zhou, H.-C.; Wei, S.-D.; Chen, J.-H. Condensed tannins from mangrove species Kandelia candel and Rhizophora mangle and their antioxidant activity. Molecules 2010, 15, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-J.; Deinzer, M.L. Tandem mass spectrometry for sequencing proanthocyanidins. Anal. Chem. 2007, 79, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Fulcrand, H.; Mané, C.; Preys, S.; Mazerolles, G.; Bouchut, C.; Mazauric, J.-P.; Souquet, J.-M.; Meudec, E.; Li, Y.; Cole, R.B. Direct mass spectrometry approaches to characterize polyphenol composition of complex samples. Phytochemistry 2008, 69, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Maldini, M.; Montoro, P.; Piacente, S.; Pizza, C. ESI-MS, ESI-MS/MS fingerprint and LC-ESI-MS analysis of proathocyanidins from Bursera simaruba Sarg bark. Nat. Prod. Commun. 2009, 4, 1671–1674. [Google Scholar] [PubMed]
- Rodrigues, C.M.; Rinaldo, D.; dos Santos, L.C.; Montoro, P.; Piacente, S.; Pizza, C.; Hiruma-Lima, C.A.; Brito, A.R.; Vilegas, W. Metabolic fingerprinting using direct flow injection electrospray ionization tandem mass spectrometry for the characterization of proanthocyanidins from the barks of Hancornia speciosa. Rapid Commun. Mass Spectrom. 2007, 21, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Tala, V.R.S.; Candida da Silva, V.; Rodrigues, C.M.; Nkengfack, A.E.; Campaner dos Santos, L.; Vilegas, W. Characterization of proanthocyanidins from parkia biglobosa (JACQ.) G. Don.(Fabaceae) by flow injection analysis—Electrospray ionization ion trap tandem mass spectrometry and liquid chromatography/electrospray ionization mass spectrometry. Molecules 2013, 18, 2803–2820. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, S.C.; Castilho, P.C. Validation of a HPLC-DAD–ESI/MS n method for caffeoylquinic acids separation, quantification and identification in medicinal Helichrysum species from Macaronesia. Food Res. Int. 2012, 45, 362–368. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Kandil, F.; Grace, M.; Seigler, D.; Cheeseman, J. Polyphenolics in Rhizophora mangle L. leaves and their changes during leaf development and senescence. Trees 2004, 18, 518–528. [Google Scholar] [CrossRef]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim. Pol. 2010, 57, 139–142. [Google Scholar] [PubMed]
- Takara, K.; Kuniyoshi, A.; Wada, K.; Kinjyo, K.; Iwasaki, H. Antioxidative flavan-3-ol glycosides from stems of Rhizophora stylosa. Biosci. Biotechnol. Biochem. 2008, 72, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Lee, S.-B.; Lee, H.-S.; Lee, S.-Y.; Baek, J.-S.; Kim, D.; Moon, T.-W.; Robyt, J.F.; Park, K.-H. Comparative study of the inhibition of α-glucosidase, α-amylase, and cyclomaltodextrin glucanosyltransferase by acarbose, isoacarbose, and acarviosine–glucose. Arch. Biochem. Biophys. 1999, 371, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Tiss, A.; Ransac, S.; Lengsfeld, H.; Hadvàry, P.; Cagna, A.; Verger, R. Surface behaviour of bile salts and tetrahydrolipstatin at air/water and oil/water interfaces. Chem. Phys. Lipids 2001, 111, 73–85. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I. Polyphenolic compounds and digestive enzymes: In vitro non-covalent interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.G.; Gonçalves, G.D.A.; Peralta, R.A.; Seixas, F.A.V.; de Sá-Nakanishi, A.B.; Bracht, L.; Comar, J.F.; Bracht, A.; Peralta, R.M. Inhibition of α-amylases by condensed and hydrolysable tannins: Focus on kinetics and hypoglycemic actions. Enzym. Res. 2017, 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Kushibiki, N.; Inagaki, Y.; Kurokawa, M.; Kawabata, J. Astilbe thunbergii reduces postprandial hyperglycemia in a type 2 diabetes rat model via pancreatic alpha-amylase inhibition by highly condensed procyanidins. Biosci. Biotechnol. Biochem. 2017, 81, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, X.; Huang, L.; Gong, H.; Cheng, B.; Sun, Y.; Li, Y.; Liu, Q.; Zheng, L.; Huang, K. Proanthocyanidins are the major anti-diabetic components of cinnamon water extract. Food Chem. Toxicol. 2013, 56, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Macarulla, M.; Aguirre, L.; Martinez-Castano, M.; Portillo, M. Quercetin can reduce insulin resistance without decreasing adipose tissue and skeletal muscle fat accumulation. Genes Nutr. 2014, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Ogura, M.; Shoji, T.; Sato, Y.; Tahara, Y.; Yamano, G.; Sato, H.; Sugizaki, K.; Fujita, N.; Tatsuoka, H. Oral administration of apple procyanidins ameliorates insulin resistance via suppression of pro-inflammatory cytokine expression in liver of diabetic ob/ob mice. J. Agric. Food Chem. 2016, 64, 8857–8865. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xu, N.; Zhao, W.; Su, J.; Liang, M.; Xie, Z.; Wu, X.; Li, Q. (−)-Epicatechin regulates blood lipids and attenuates hepatic steatosis in rats fed high-fat diet. Mol. Nutr. Food Res. 2017, 61, 1700303. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Burrington, C.M.; Graff, E.C.; Zhang, J.; Judd, R.L.; Suksaranjit, P.; Kaewpoowat, Q.; Davenport, S.K.; O’Neill, A.M.; Greene, M.W. Metabolic phenotype and adipose and liver features in a high-fat Western diet-induced mouse model of obesity-linked NAFLD. Am. J. Physiol. Endocrinol. Metab. 2015, 310, E418–E439. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol. Metab. 2008, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Koonen, D.P.; Jacobs, R.L.; Febbraio, M.; Young, M.E.; Soltys, C.-L.M.; Ong, H.; Vance, D.E.; Dyck, J.R. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes 2007, 56, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-W.; Jiang, Y.; Zhang, D.-Y.; Zhang, X.-J.; Hu, Y.-J.; Li, P.; Su, H.; Wan, J.-B. The hepatoprotective effect of aqueous extracts of Penthorum chinense Pursh against acute alcohol-induced liver injury is associated with ameliorating hepatic steatosis and reducing oxidative stress. Food Funct. 2015, 6, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Im, A.-R.; Kim, Y.H.; Lee, H.W.; Song, K.H. Water extract of Dolichos lablab attenuates hepatic lipid accumulation in a cellular nonalcoholic fatty liver disease model. J. Med. Food 2016, 19, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Pisonero-Vaquero, S.; Martínez-Ferreras, Á.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Fernández, A.; Benet, M.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; Sánchez-Campos, S. Quercetin ameliorates dysregulation of lipid metabolism genes via the PI3K/AKT pathway in a diet-induced mouse model of nonalcoholic fatty liver disease. Mol. Nutr. Food Res. 2015, 59, 879–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Kim, J.H.; Jang, Y.S.; Kim, C.H.; Lee, J.-Y.; Lim, S.S. Anti-obesity effect of Solidago virgaurea var. g igantea extract through regulation of adipogenesis and lipogenesis pathways in high-fat diet-induced obese mice (C57BL/6N). Food Nutr. Res. 2017, 61, 1273479. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.J.; Jegal, J.; Ahn, J.; Kim, J.; Yang, M.H. Anti-obesity effect of Dioscorea oppositifolia extract in high-fat diet-induced obese mice and its chemical characterization. Boil. Pharm. Bull. 2016, 39, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhao, M.; Wang, M.; Yan, W.; Liu, Y.; Ren, S.; Lu, J.; Wang, B.; Chen, L. Effects of canagliflozin on weight loss in high-fat diet-induced obese mice. PLoS ONE 2017, 12, e0179960. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yu, Z.; Tang, Q.; Song, H.; Gao, Z.; Chen, W.; Zheng, X. Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct. 2013, 4, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-Y.; Kang, M.-J.; Choe, E.; Kim, J.-I. Hypoglycemic and antioxidant effects of Daraesoon (Actinidia arguta shoot) in animal models of diabetes mellitus. Nutr. Res. Pr. 2015, 9, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-P.; Su, C.-H.; Lu, T.-M.; Lai, M.-N.; Ng, L.-T. Effects of Grifola frondosa non-polar bioactive components on high-fat diet fed and streptozotocin-induced hyperglycemic mice. Pharm. Boil. 2015, 53, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Vazquez Prieto, M.A.; Bettaieb, A.; Rodriguez Lanzi, C.; Soto, V.C.; Perdicaro, D.J.; Galmarini, C.R.; Haj, F.G.; Miatello, R.M.; Oteiza, P.I. Catechin and quercetin attenuate adipose inflammation in fructose-fed rats and 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2015, 59, 622–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draper, H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Ezymol. 1990, 86, 421–431. [Google Scholar]
- Hamed, E.A.; Zakary, M.M.; Ahmed, N.S.; Gamal, R.M. Circulating leptin and insulin in obese patients with and without type 2 diabetes mellitus: Relation to ghrelin and oxidative stress. Diabetes Res. Clin. Pract. 2011, 94, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Karczewska-Kupczewska, M.; Strączkowski, M.; Adamska, A.; Nikołajuk, A.; Otziomek, E.; Górska, M.; Kowalska, I. Increased suppression of serum ghrelin concentration by hyperinsulinemia in women with anorexia nervosa. Eur. J. Endocrinol. 2010, 162, 235–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, Y.; Okabe, M.; Natsume, M.; Ashida, H. Cinnamtannin A2, a tetrameric procyanidin, increases GLP-1 and insulin secretion in mice. Biosci. Biotechnol. Biochem. 2013, 77, 888–891. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira, C.C.; Acedo, S.C.; Gotardo, É.M.F.; de Oliveira Carvalho, P.; Rocha, T.; Pedrazzoli, J.; Gambero, A. Effects of methotrexate on inflammatory alterations induced by obesity: An in vivo and in vitro study. Mol. Cell. Endocrinol. 2012, 361, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Nakamitsu, P.Z.; Compri, C.M.; de Fraia, P.L.; Gotardo, E.M.; de Oliveira, C.C.; Ribeiro, M.L.; Pedrazzoli, J., Jr.; Gambero, A. Thalidomide controls adipose tissue inflammation associated with high-fat diet-induced obesity in mice. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 151–158. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |











| m/z [M − H]− | MS² | MS³ | Proposed Name |
|---|---|---|---|
| 289 | 137 [M-152-H]− | catechin | |
| 435 | 283 [M-152-H]− | 137 [M-152-146-H]− | catechin + deoxyhexose |
| 451 | 299 [M-152-H]− | 137 [M-152-162-H]− | catechin + hexose |
| 515 | 353 [M-162-H]− | 191 [M-162-162-H]− | dicaffeoyl-quinic acid |
| 577 | 451 [M-126-H]− | catechin dimer | |
| 425 [M-152-H]− | |||
| 289 [M-288-H]− | |||
| 609 | 463 [M-146-H]− | rutin | |
| 301 [M-308-H]− | |||
| 723 | 571 [M-152-H]− | 419 [M-152-H]− | catechin dimer + deoxyhexose |
| 739 | 587 [M-152-H]− | 569 [M-18-H]− | catechin dimer + hexose |
| 435 [M-152-H]− | |||
| 865 | 577 [M-288-H]− | 451 [M-126-H]− | catechin trimer |
| 425 [M-152-H]− | |||
| 289 [M-288-H]− | |||
| 1153 | 865 [M-288-H]− | 847 [M-18-H]− | catechin tetramer |
| 739 [M-126-H]− | |||
| 587 [M-278-H]− | |||
| 577 [M-288-H]− | |||
| 451 [M-414-H]− |
| Parameters | Control | Control5 | Control50 | HFD | HFD5 | HFD50 |
|---|---|---|---|---|---|---|
| Body weight at 8th week (g) | 42.2 ± 1.4 | 41.7 ± 1.2 | 43.0 ± 1.8 | 55.0 ± 1.2 * | 52.0 ± 2.0 | 53.0 ± 1.2 |
| Final body weight (g) | 44.7 ± 1.8 | 45.2 ± 1.6 | 43.5 ± 1.3 | 61.2 ± 2.4 * | 56.0 ± 2.7 | 55.2 ± 1.3 |
| ∆ Body weight (%) | 5.8 ± 1.2 | 8.2 ± 1.5 | 1.6 ± 2.5 | 9.8 ± 2.6 | 6.2 ± 2.7 | 4.2 ± 2.2 |
| Food intake (kcal/day) | 14.3 ± 1.1 | 13.3 ± 0.4 | 12.0 ± 0.4 # | 25.5 ± 1.1 | 24.3 ± 0.9 | 23.4 ± 1.9 |
| Epididimal fat (g) | 1.8 ± 0.3 | 1.5 ± 0.2 | 1.5 ± 0.1 | 2.7 ± 0.1 * | 2.9 ± 0.2 | 2.7 ± 0.4 |
| Epididimal fat (%) a | 4.0 ± 0.4 | 3.4 ± 0.3 | 3.7 ± 0.1 | 4.4 ± 0.1 | 5.1 ± 0.4 | 5.1 ± 0.8 |
| Subcutaneous fat (g) | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 1.3 ± 0.1 * | 1.0 ± 0.1 | 1.2 ± 0.2 |
| Subcutaneous fat (%) a | 1.6 ± 0.2 | 1.2 ± 0.1 | 1.5 ± 0.1 | 2.1 ± 0.1 * | 1.8 ± 0.3 | 2.2 ± 0.2 |
| Liver (g) | 1.8 ± 0.1 | 1.9 ± 0.1 | 1.7 ± 0.1 | 3.0 ± 0.3 * | 2.1 ± 0.1 # | 2.2 ± 0.2 # |
| Liver (%) a | 4.2 ± 0.2 | 4.2 ± 0.1 | 4.0 ± 0.1 | 4.9 ± 0.3 | 3.7 ± 0.3 # | 3.9 ± 0.1 # |
| Gastrocnemius muscle (g) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| Gastrocnemius muscle (%) a | 0.5 ± 0.1 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 |
| Parameters | Control | Control5 | Control50 | HFD | HFD5 | HFD50 |
|---|---|---|---|---|---|---|
| Fasting glucose (mg/dL) | 166 ± 5 | 150 ± 4 | 152 ± 5 | 226 ± 11 * | 201 ± 22 | 197 ± 6 # |
| Fasting insulin (ng/mL) | 106 ± 13 | 112 ± 14 | 207 ± 16 # | 176 ± 34 * | 161 ± 17 | 127 ± 9 # |
| kITT | 5.4 ± 0.3 | 5.7 ± 0.7 | 5.0 ± 0.5 | 2.0 ± 0.4 * | 2.6 ± 0.8 | 3.2 ± 0.3 # |
| Total cholesterol (mg/dL) | 174 ± 2 | 164 ± 2 # | 168 ± 2 | 203 ± 9 * | 184 ± 7 | 180 ± 4 # |
| LDL-cholesterol (mg/dL) | 100 ± 7 | 98 ± 6 | 102 ± 6 | 144 ± 10 * | 118 ± 4 # | 106 ± 9 # |
| HDL-cholesterol (mg/dL) | 57 ± 4 | 54 ± 3 | 52 ± 1 | 52 ± 2 | 50 ± 1 | 54 ± 2 |
| Triglycerides (mg/dL) | 187 ± 22 | 193 ± 32 | 201 ± 24 | 99 ± 7 * | 98 ± 5 | 127 ± 17 |
| Gene | Primer | Sequence (5′ → 3′) |
|---|---|---|
| Pparg | Sense | GATGGAAGACCACTCGCATT |
| Antisense | AACCATTGGGTCAGCTCTTG | |
| Ppara | Sense | AGAAGTTGCAGGAGGGGATT |
| Antisense | TTGAAGCAGCTTYGGGAAGA | |
| Srebf1 | Sense | GTGAGCCTGACAAGCAATCA |
| Antisense | GGTGCCTACAGAGCAAGAGG | |
| Cd36 | Sense | ATTCTCATGCCAGTCGGAGA |
| Antisense | TGGCTTTTGCACATCAAAGA | |
| Tnf | Sense | TAGCCAGGAGGGAGAACAGA |
| Antisense | TTTTCTGGAGGGAGATGTGG | |
| Ccl2 | Sense | CCCAATGAGTAGGCTGGAGA |
| Antisense | TCTGGACCCATTCCTTCTTG | |
| Lep | Sense | CTATGCCACCTTGGTCACCT |
| Antisense | ACCAAACCAAGCATTTTTGC | |
| Il10 | Sense | ATCGATTTCTCCCCTGTGAA |
| Antisense | TTCATGGCCTTGTAGACACCT | |
| Il6 | Sense | TCTCTGGGAAATCGTGGAA |
| Antisense | TTCTGCAAGTGCATCATCG | |
| Fasn | Sense | CACAGATGATGACAGGAGATGGA |
| Antisense | TCGGAGTGAGGCTGGGTTGATA | |
| 18s | Sense | AAACGGCTACCACATCCAAG |
| Antisense | CAATTACAGGGCCTCGAAAG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Souza Mesquita, L.M.; Caria, C.R.e.P.; Santos, P.S.; Ruy, C.C.; Da Silva Lima, N.; Moreira, D.K.T.; Da Rocha, C.Q.; Murador, D.C.; De Rosso, V.V.; Gambero, A.; et al. Modulatory Effect of Polyphenolic Compounds from the Mangrove Tree Rhizophora mangle L. on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in High-Fat Diet Obese Mice. Molecules 2018, 23, 2114. https://doi.org/10.3390/molecules23092114
De Souza Mesquita LM, Caria CReP, Santos PS, Ruy CC, Da Silva Lima N, Moreira DKT, Da Rocha CQ, Murador DC, De Rosso VV, Gambero A, et al. Modulatory Effect of Polyphenolic Compounds from the Mangrove Tree Rhizophora mangle L. on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in High-Fat Diet Obese Mice. Molecules. 2018; 23(9):2114. https://doi.org/10.3390/molecules23092114
Chicago/Turabian StyleDe Souza Mesquita, Leonardo Mendes, Cíntia Rabelo e Paiva Caria, Paola Souza Santos, Caio Cesar Ruy, Natalia Da Silva Lima, Débora Kono Taketa Moreira, Claudia Quintino Da Rocha, Daniella Carisa Murador, Veridiana Vera De Rosso, Alessandra Gambero, and et al. 2018. "Modulatory Effect of Polyphenolic Compounds from the Mangrove Tree Rhizophora mangle L. on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in High-Fat Diet Obese Mice" Molecules 23, no. 9: 2114. https://doi.org/10.3390/molecules23092114