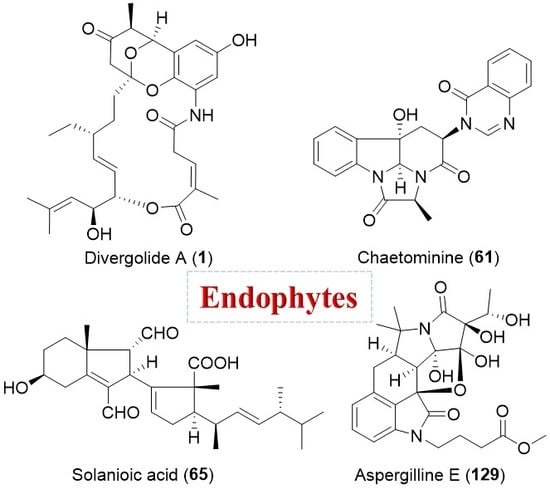
Structural Diversity and Biological Activities of Novel Secondary Metabolites from Endophytes
Abstract
:1. Introduction
2. Polyketides
2.1. Macrolides
2.2. Benzopyran
2.3. Spiro Compounds
2.4. Quinones
2.5. Nitrogen-Containing Heterocycles
2.6. Others
3. Noribosomal Peptides
4. Isoprenoids
4.1. Steroids
4.2. Sesquiterpenoids
4.3. Diterpenoids
4.4. Sesterterpenoids
5. Hybrid Products
5.1. PKS-NRPS
5.2. NRPS-Terpene
5.3. PKS-Terpene
5.4. PKS-NRPS-Terpene
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Morris-Natschke, S.L.; Lee, K.H. Strategies for the optimization of natural leads to anticancer drugs or drug candidates. Med. Res. Rev. 2016, 36, 32–91. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Van Drie, J.H. Generation of three-dimensional pharmacophore models. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 449–464. [Google Scholar] [CrossRef]
- Butler, M.S.; Robertson, A.A.; Cooper, M.A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014, 31, 1612–1661. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lou, H.X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, P. Chemical ecology of fungi. Nat. Prod. Rep. 2015, 32, 971–993. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schaberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Spiteller, M. Are we ready for industrial production of bioactive plant secondary metabolites utilizing endophytes? Nat. Prod. Rep. 2011, 28, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Pandey, S.P.; Spiteller, M. Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochemistry 2013, 91, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Chen, L.; Xin, H.L.; Zheng, C.J.; Rahman, K.; Han, T.; Qin, L.P. A friendly relationship between endophytic fungi and medicinal plants: A systematic review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef] [PubMed]
- Scherlach, K.; Hertweck, C. Mediators of mutualistic microbe-microbe interactions. Nat. Prod. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lyons, P.C.; Plattner, R.D.; Bacon, C.W. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 1986, 232, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Q.Y.; Jia, M.; Ming, Q.L.; Yue, W.; Rahman, K.; Qin, L.P.; Han, T. Endophytic fungi with antitumor activities: Their occurrence and anticancer compounds. Crit. Rev. Microbiol. 2016, 42, 454–473. [Google Scholar] [CrossRef] [PubMed]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Endophytic and epiphytic microbes as “sources” of bioactive agents. Front. Chem. 2015, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Verekar, S.A.; Bhave, S.V. Endophytic fungi: A reservoir of antibacterials. Front. Microbiol. 2015, 5, 715. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes—Secret producers of bioactive plant metabolites. Pharmazie 2013, 68, 499–505. [Google Scholar] [PubMed]
- Radic, N.; Strukelj, B. Endophytic fungi: The treasure chest of antibacterial substances. Phytomedicine 2012, 19, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites. Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Maier, A.; Fiebig, H.H.; Gorls, H.; Lin, W.H.; Peschel, G.; Hertweck, C. Divergolides A–D from a mangrove endophyte reveal an unparalleled plasticity in ansa-macrolide biosynthesis. Angew. Chem. Int. Ed. 2011, 50, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Li, S.R.; Zhao, G.S.; Sun, M.W.; He, H.G.; Wang, H.X.; Li, Y.Y.; Lu, C.H.; Shen, Y.M. Identification and characterization of the biosynthetic gene cluster of divergolides from Streptomyces sp. W112. Gene 2014, 544, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.L.; Ding, L.; Hertweck, C. A branched extender unit shared between two orthogonal polyketide pathways in an endophyte. Angew. Chem. Int. Ed. 2011, 50, 4667–4670. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Franke, J.; Hertweck, C. Divergolide congeners illuminate alternative reaction channels for ansamycin diversification. Org. Biomol. Chem. 2015, 13, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Rasapalli, S.; Jarugumilli, G.; Yarrapothu, G.R.; Golen, J.A.; Rheingold, A.L. Synthesis of the naphthoquinone core of divergolides (C–D) and model studies for elaboration of the ansabridge. Tetrahedron Lett. 2013, 54, 2615–2618. [Google Scholar] [CrossRef]
- Nawrat, C.C.; Kitson, R.R.; Moody, C.J. Toward the total synthesis of hygrocin B and divergolide C: Construction of the naphthoquinone-azepinone core. Org. Lett. 2014, 16, 1896–1899. [Google Scholar] [CrossRef] [PubMed]
- Inahashi, Y.; Iwatsuki, M.; Ishiyama, A.; Matsumoto, A.; Hirose, T.; Oshita, J.; Sunazuka, T.; Panbangred, W.; Takahashi, Y.; Kaiser, M.; et al. Actinoallolides A–E, new anti-trypanosomal macrolides, produced by an endophytic actinomycete, Actinoallomurus fulvus MK10-036. Org. Lett. 2015, 17, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, A.S.; Yoshida, W.Y.; Mooberry, S.L.; Hemscheidt, T. The structure of microcarpalide, a microfilament disrupting agent from an endophytic fungus. Org. Lett. 2001, 3, 3479–3481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Krohn, K.; Zia, U.; Florke, U.; Pescitelli, G.; Di Bari, L.; Antus, S.; Kurtan, T.; Rheinheimer, J.; Draeger, S.; et al. New mono- and dimeric members of the secalonic acid family: Blennolides A–G isolated from the fungus Blennoria sp. Chem. Eur. J. 2008, 14, 4913–4923. [Google Scholar] [CrossRef] [PubMed]
- Pontius, A.; Krick, A.; Kehraus, S.; Foegen, S.E.; Muller, M.; Klimo, K.; Gerhauser, C.; Konig, G.M. Noduliprevenone: A novel heterodimeric chromanone with cancer chemopreventive potential. Chem. Eur. J. 2008, 14, 9860–9863. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.; Kouam, S.F.; Kuigoua, G.M.; Hussain, H.; Cludius-Brandt, S.; Florke, U.; Kurtan, T.; Pescitelli, G.; Di Bari, L.; Draeger, S.; et al. Xanthones and oxepino[2,3-b]chromones from three endophytic fungi. Chem. Eur. J. 2009, 15, 12121–12132. [Google Scholar] [CrossRef] [PubMed]
- Lösgen, S.; Magull, J.; Schulz, B.; Draeger, S.; Zeeck, A. Isofusidienols: Novel chromone-3-oxepines produced by the endophytic fungus Chalara sp. Eur. J. Org. Chem. 2008, 2008, 698–703. [Google Scholar] [CrossRef]
- Li, C.; Yang, B.; Fenstemacher, R.; Turkson, J.; Cao, S. Lycopodiellactone, an unusual δ-lactone-isochromanone from a Hawaiian plant-associated fungus Paraphaeosphaeria neglecta FT462. Tetrahedron Lett. 2015, 56, 1724–1727. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Si, Y.K.; Jiang, X.J.; Guo, L.D.; Che, Y.S. Virgatolides A–C, benzannulated spiroketals from the plant endophytic fungus Pestalotiopsis virgatula. Org. Lett. 2011, 13, 2670–2673. [Google Scholar] [CrossRef] [PubMed]
- Kesting, J.R.; Staerk, D.; Tejesvi, M.V.; Kini, K.R.; Prakash, H.S.; Jaroszewski, J.W. HPLC-SPE-NMR identification of a novel metabolite containing the benzo[c]oxepin skeleton from the endophytic fungus Pestalotiopsis virgatula culture. Planta Med. 2009, 75, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Kesting, J.R.; Olsen, L.; Staerk, D.; Tejesvi, M.V.; Kini, K.R.; Prakash, H.S.; Jaroszewski, J.W. Production of unusual dispiro metabolites in Pestalotiopsis virgatula endophyte cultures: HPLC-SPE-NMR, electronic circular dichroism, and time-dependent density-functional computation study. J. Nat. Prod. 2011, 74, 2206–2215. [Google Scholar] [CrossRef] [PubMed]
- Badrinarayanan, S.; Squire, C.J.; Sperry, J.; Brimble, M.A. Bioinspired total synthesis and stereochemical revision of the fungal metabolite pestalospirane B. Org. Lett. 2017, 19, 3414–3417. [Google Scholar] [CrossRef] [PubMed]
- Van der Sar, S.A.; Blunt, J.W.; Munro, M.H. spiro-Mamakone A: A unique relative of the spirobisnaphthalene class of compounds. Org. Lett. 2006, 8, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Van der Sar, S.A.; Lang, G.; Mitova, M.I.; Blunt, J.W.; Cole, A.L.; Cummings, N.; Ellis, G.; Munro, M.H. Biosynthesis of spiro-mamakone A, a structurally unprecedented fungal metabolite. J. Org. Chem. 2008, 73, 8635–8638. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.C.; Devenish, S.R.; Muscroft-Taylor, A.C.; Blunt, J.W.; Munro, M.H. Synthetic and biological studies on the spiro-mamakone system. Org. Biomol. Chem. 2008, 6, 3854–3862. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, G.; Li, Y.; Qu, J.; Ma, S.; Lv, H.; Liu, Y.; Wang, W.; Dai, J.; Tang, Y.; Yu, S. Structures and absolute configurations of penicillactones A–C from an endophytic microorganism, Penicillium dangeardii Pitt. Org. Lett. 2013, 15, 5206–5209. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Yang, X.S.; Schwartz, M.; Strobel, G.; Clardy, J. The Relationship between an Endangered North-American Tree and an Endophytic Fungus. Chem. Biol. 1995, 2, 721–727. [Google Scholar] [CrossRef]
- Lee, J.C.; Strobel, G.A.; Lobkovsky, E.; Clardy, J. Torreyanic acid: A selectively cytotoxic quinone dimer from the endophytic fungus Pestalotiopsis microspora. J. Org. Chem. 1996, 61, 3232–3233. [Google Scholar] [CrossRef]
- Li, C.; Johnson, R.P.; Porco, J.A. Total synthesis of the quinone epoxide dimer (+)-torreyanic acid: Application of a biomimetic oxidation/electrocyclization/Diels-Alder dimerization cascade. J. Am. Chem. Soc. 2003, 125, 5095–5106. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Pan, S.C. Total synthesis of the novel, biologically active epoxyquinone dimer (+/−)-torreyanic acid: A biomimetic approach. Org. Lett. 2004, 6, 3985–3988. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Li, Y.; Fu, S.; Liu, S.; Wei, J.; Che, Y. Ambuic acid and torreyanic acid derivatives from the endolichenic fungus Pestalotiopsis sp. J. Nat. Prod. 2009, 72, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.M.; Yang, S.X.; Qin, J.C. Azaphilones: Chemistry and biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Song, Y.C.; Chen, J.R.; Xu, C.; Ge, H.M.; Wang, X.T.; Tan, R.X. Chaetoglobosin U, a cytochalasan alkaloid from endophytic Chaetomium globosum IFB-E019. J. Nat. Prod. 2006, 69, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Ming Ge, H.; Yun Zhang, W.; Ding, G.; Saparpakorn, P.; Song, Y.C.; Hannongbua, S.; Tan, R.X. Chaetoglobins A and B, two unusual alkaloids from endophytic Chaetomium globosum culture. Chem. Commun. 2008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Jiang, N.; Gu, W.; Ma, J.; Wang, Y.R.; Song, Y.C.; Tan, R.X. Characterization, synthesis and self-aggregation of (−)-alternarlactam: A new fungal cytotoxin with cyclopentenone and isoquinolinone scaffolds. Chem. Eur. J. 2010, 16, 14479–14485. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-X.; Xiao, J.; Laatsch, H.; Holstein, J.J.; Dittrich, B.; Zhang, Q.; Gao, J.-M. Fusarimine, a novel polyketide isoquinoline alkaloid, from the endophytic fungus Fusarium sp. LN12, isolated from Melia azedarach. Tetrahedron Lett. 2012, 53, 6372–6375. [Google Scholar] [CrossRef]
- Cao, P.; Yang, J.; Miao, C.P.; Yan, Y.; Ma, Y.T.; Li, X.N.; Zhao, L.X.; Huang, S.X. New duclauxamide from Penicillium manginii YIM PH30375 and structure revision of the duclauxin family. Org. Lett. 2015, 17, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Elsebai, M.F.; Saleem, M.; Tejesvi, M.V.; Kajula, M.; Mattila, S.; Mehiri, M.; Turpeinen, A.; Pirttila, A.M. Fungal phenalenones: Chemistry, biology, biosynthesis and phylogeny. Nat. Prod. Rep. 2014, 31, 628–645. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ma, Y.T.; Yang, J.; Horsman, G.P.; Luo, D.; Ji, X.; Huang, S.X. Tropolone ring construction in the biosynthesis of rubrolone B, a cationic tropolone alkaloid from endophytic Streptomyces. Org. Lett. 2016, 18, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Palleroni, N.J.; Reichelt, K.E.; Mueller, D.; Epps, R.; Tabenkin, B.; Bull, D.N.; Schuep, W.; Berger, J. Production of a novel red pigment, rubrolone, by Streptomyces echinoruber sp. nov. I. taxonomy, fermentation and partial purification. J. Antibiot. 1978, 31, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Schuep, W.; Blount, J.F.; Williams, T.H.; Stempel, A. Production of a novel red pigment, rubrolone, by Streptomyces echinoruber Sp. nov. II. chemistry and structure elucidation. J. Antibiot. 1978, 31, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Boyle, C.; Draeger, S.; Rommert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Krohn, K.; Biele, C.; Drogies, K.H.; Steingrover, K.; Aust, H.J.; Draeger, S.; Schulz, B. Fusidilactones, a new group of polycyclic lactones from an endophyte, Fusidium sp. Eur. J. Org. Chem. 2002. [CrossRef]
- Qin, S.; Krohn, K.; Flörke, U.; Schulz, B.; Draeger, S.; Pescitelli, G.; Salvadori, P.; Antus, S.; Kurtán, T. Two new fusidilactones from the fungal endophyte Fusidium sp. Eur. J. Org. Chem. 2009, 2009, 3279–3284. [Google Scholar] [CrossRef]
- Wang, J.S.; Hsung, R.P.; Ghosh, S.K. Stereoselective ketal-tethered intramolecular Diels-Alder cycloadditions. An approach to the 2-oxadecalin spiroketal core of antifungal agent fusidilactione C. Org. Lett. 2004, 6, 1939–1942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Huang, W.Y.; Chen, J.R.; Yan, W.Z.; Xie, D.Q.; Tan, R.X. Cephalosol: An antimicrobial metabolite with an unprecedented skeleton from endophytic Cephalosporium acremonium IFB-E007. Chem. Eur. J. 2008, 14, 10670–10674. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, T.; Takenaka, Y.; Nagakura, N.; Hamada, N. 6H-dibenzo[b,d]pyran-6-one derivatives from the cultured lichen mycobionts of Graphis spp. and their biosynthetic origin. Phytochemistry 2003, 62, 71–75. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, N.; Cheng, B.; Zhai, H. Total synthesis of (+/−)-cephalosol. Org. Lett. 2012, 14, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Luo, D.; Yamashita, K.; Oshima, Y. Structures and biomimetic synthesis of novel alpha-pyrone polyketides of an endophytic Penicillium sp. in Catharanthus roseus. Org. Lett. 2013, 15, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Eade, S.J.; Walter, M.W.; Byrne, C.; Odell, B.; Rodriguez, R.; Baldwin, J.E.; Adlington, R.M.; Moses, J.E. Biomimetic synthesis of pyrone-derived natural products: Exploring chemical pathways from a unique polyketide precursor. J. Org. Chem. 2008, 73, 4830–4839. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.C.; Jumpathong, J.; Lumyong, S.; Voigt, K.; Hertweck, C. Daldionin, an unprecedented binaphthyl derivative, and diverse polyketide congeners from a fungal orchid endophyte. Chem. Eur. J. 2016, 22, 4551–4555. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Hussain, H.; Ahmed, I.; Draeger, S.; Schulz, B.; Meier, K.; Steinert, M.; Pescitelli, G.; Kurtán, T.; Flörke, U.; et al. Viburspiran, an antifungal member of the octadride class of maleic anhydride natural products. Eur. J. Org. Chem. 2011, 2011, 808–812. [Google Scholar] [CrossRef]
- Ebrahim, W.; Aly, A.H.; Wray, V.; Proksch, P.; Debbab, A. Unusual octalactones from Corynespora cassiicola, an endophyte of Laguncularia racemosa. Tetrahedron Lett. 2013, 54, 6611–6614. [Google Scholar] [CrossRef]
- Zhu, T.J.; Du, L.; Hao, P.F.; Lin, Z.J.; Gu, Q.Q. Citrinal A, a novel tricyclic derivative of citrinin, from an algicolous fungus Penicillium sp. i-1-1. Chin. Chem. Lett. 2009, 20, 917–920. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Ma, H.; Jiang, J.; Sun, W.; Cheng, L.; Zhang, G.; Zhang, Y. Citrinal B, a new secondary metabolite from endophytic fungus Colletotrichum capsici and structure revision of citrinal A. Tetrahedron Lett. 2016, 57, 4250–4253. [Google Scholar] [CrossRef]
- Brady, S.F.; Singh, M.P.; Janso, J.E.; Clardy, J. Cytoskyrins A and B, new BIA active bisanthraquinones isolated from an endophytic fungus. Org. Lett. 2000, 2, 4047–4049. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Papageorgiou, C.D.; Piper, J.L.; Chadha, R.K. The cytoskyrin cascade: A facile entry into cytoskyrin A, deoxyrubroskyrin, rugulin, skyrin, and flavoskyrin model systems. Angew. Chem. Int. Ed. 2005, 44, 5846–5851. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Lim, Y.H.; Papageorgiou, C.D.; Piper, J.L. Total synthesis of (+)-rugulosin and (+)-2,2′-epi-cytoskyrin A through cascade reactions. Angew. Chem. Int. Ed. 2005, 44, 7917–7921. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Lim, Y.H.; Piper, J.L.; Papageorgiou, C.D. Total syntheses of 2,2′-epi-cytoskyrin A, rugulosin, and the alleged structure of rugulin. J. Am. Chem. Soc. 2007, 129, 4001–4013. [Google Scholar] [CrossRef] [PubMed]
- Lhamo, S.; Wang, X.-B.; Li, T.-X.; Wang, Y.; Li, Z.-R.; Shi, Y.-M.; Yang, M.-H.; Kong, L.-Y. Three unusual indole diketopiperazine alkaloids from a terrestrial-derived endophytic fungus, Aspergillus sp. Tetrahedron Lett. 2015, 56, 2823–2826. [Google Scholar] [CrossRef]
- Meng, L.H.; Wang, C.Y.; Mandi, A.; Li, X.M.; Hu, X.Y.; Kassack, M.U.; Kurtan, T.; Wang, B.G. Three diketopiperazine alkaloids with spirocyclic skeletons and one bisthiodiketopiperazine derivative from the mangrove-derived endophytic fungus Penicillium brocae MA-231. Org. Lett. 2016, 18, 5304–5307. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhou, G.; Zhu, M.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Neosartoryadins A and B, fumiquinazoline alkaloids from a mangrove-derived fungus Neosartorya udagawae HDN13-313. Org. Lett. 2016, 18, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Xu, M.Y.; Li, H.J.; Li, J.Q.; Chen, Y.X.; Ma, W.Z.; Li, Y.P.; Xu, J.; Yang, D.P.; Lan, W.J. Amino acid-directed strategy for inducing the marine-derived fungus Scedosporium apiospermum F41-1 to maximize alkaloid diversity. Org. Lett. 2017, 19, 4888–4891. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.H.; Xu, S.; Liu, J.Y.; Ge, H.M.; Ding, H.; Xu, C.; Zhu, H.L.; Tan, R.X. Chaetominine, a cytotoxic alkaloid produced by endophytic Chaetomium sp. IFB-E015. Org. Lett. 2006, 8, 5709–5712. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Kuo, J.; Yoshida, W.Y.; Kelly, M.; Scheuer, P.J. More kapakahines from the marine sponge Cribrochalina olemda. Org. Lett. 2003, 5, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liang, K.; Tong, X.; Ding, M.; Li, D.; Xia, C. Biomimetic synthesis of (−)-chaetominine epimers via copper-catalyzed radical cyclization. Tetrahedron 2015, 71, 3699–3704. [Google Scholar] [CrossRef]
- DarkinRattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bednarek, M.A.; et al. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 1996, 93, 13143–13147. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Liesch, J.M.; Dombrowski, A.W.; Darkin-Rattray, S.J.; Schmatz, D.M.; Goetz, M.A. Structure, histone deacetylase, and antiprotozoal activities of apicidins B and C, congeners of apicidin with proline and valine substitutions. Org. Lett. 2001, 3, 2815–2818. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Ahn, J.W.; Kim, H.S.; Lee, J.; Yoon, J.H. Apicidin inhibits cell growth by downregulating IGF-1R in salivary mucoepidermoid carcinoma cells. Oncol. Rep. 2015, 33, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Bauden, M.; Tassidis, H.; Ansari, D. In vitro cytotoxicity evaluation of HDAC inhibitor apicidin in pancreatic carcinoma cells subsequent time and dose dependent treatment. Toxicol. Lett. 2015, 236, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lai, Z.; Huang, W.; Ling, H.; Lin, M.; Tang, S.; Liu, Y.; Tao, Y. Apicidin inhibited proliferation and invasion and induced apoptosis via mitochondrial pathway in non-small cell lung cancer GLC-82 Cells. Anticancer Agents Med. Chem. 2017, 17, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Ratnaweera, P.B.; Williams, D.E.; Patrick, B.O.; de Silva, E.D.; Andersen, R.J. Solanioic acid, an antibacterial degraded steroid produced in culture by the fungus Rhizoctonia solani isolated from tubers of the medicinal plant Cyperus rotundus. Org. Lett. 2015, 17, 2074–2077. [Google Scholar] [CrossRef] [PubMed]
- Wipf, P.; Halter, R.J. Chemistry and biology of wortmannin. Org. Biomol. Chem. 2005, 3, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Ross, L.; Tamayo, G.; Clardy, J. Asterogynins: Secondary metabolites from a Costa Rican endophytic fungus. Org. Lett. 2010, 12, 4661–4663. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.-E.; Yang, Z.-D.; Sheng, L.; Zhou, S.-Y.; Li, S.; Yao, X.-J.; Zhi, K.-K.; Wang, Y.-G.; Zhang, F. Secovironolide, a novel furanosteroid scaffold with a five-membered B ring from the endophytic fungus Talaromyces wortmannii LGT-4. Tetrahedron Lett. 2015, 56, 6754–6757. [Google Scholar] [CrossRef]
- Fu, G.-C.; Yang, Z.-D.; Zhou, S.-Y.; Li, X.-M.; Yu, H.-T.; Yao, X.-J.; Fang, J.-G.; Shu, Z.-M.; Xue, H.-Y.; Wang, Y.-G. Wortmannines A–C, three novel wortmannin derivatives with an unusual five-membered B ring from the endophytic fungus Talaromyces wortmannii LGT-4. Tetrahedron Lett. 2016, 57, 4608–4611. [Google Scholar] [CrossRef]
- Suzuki, T.; Kobayashi, S. Concise approach to pupukeanane skeleton: Synthetic study of chloropupukeananin. Org. Lett. 2010, 12, 2920–2923. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, S.; Jiang, L.; Chen, X.; Guo, L.; Che, Y. Chloropupukeananin, the first chlorinated pupukeanane derivative, and its precursors from Pestalotiopsis fici. Org. Lett. 2008, 10, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miyajima, Y.; Suzuki, K.; Iwakiri, K.; Koshimizu, M.; Hirai, G.; Sodeoka, M.; Kobayashi, S. Unexpected Diels-Alder/carbonyl-ene cascade toward the biomimetic synthesis of chloropupukeananin. Org. Lett. 2013, 15, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Liu, S.C.; Zheng, Z.H.; Chen, X.L.; Zhang, H.; Guo, L.D.; Che, Y.S. Chloropestolide A, an antitumor metabolite with an unprecedented spiroketal skeleton from Pestalotiopsis fici. Org. Lett. 2009, 11, 2836–2839. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Niu, S.; Lu, X.; Chen, X.; Zhang, H.; Guo, L.; Che, Y. Unique metabolites of Pestalotiopsis fici suggest a biosynthetic hypothesis involving a Diels-Alder reaction and then mechanistic diversification. Chem. Commun. 2010, 46, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bruhn, T.; Guo, L.; Gotz, D.C.; Brun, R.; Stich, A.; Che, Y.; Bringmann, G. Chloropupukeanolides C-E: Cytotoxic pupukeanane chlorides with a spiroketal skeleton from Pestalotiopsis fici. Chem. Eur. J. 2011, 17, 2604–2613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ge, H.; Zou, J.H.; Tao, X.; Chen, R.; Dai, J. Periconianone A, a new 6/6/6 carbocyclic sesquiterpenoid from endophytic fungus Periconia sp. with neural anti-inflammatory activity. Org. Lett. 2014, 16, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Liffert, R.; Linden, A.; Gademann, K. Total synthesis of the sesquiterpenoid periconianone a based on a postulated biogenesis. J. Am. Chem. Soc. 2017, 139, 16096–16099. [Google Scholar] [CrossRef] [PubMed]
- Pulici, M.; Sugawara, F.; Koshino, H.; Uzawa, J.; Yoshida, S.; Lobkovsky, E.; Clardy, J. Pestalotiopsins A and B: New caryophyllenes from an endophytic fungus of Taxus brevifolia. J. Org. Chem. 1996, 61, 2122–2124. [Google Scholar] [CrossRef]
- Ding, L.; Goerls, H.; Dornblut, K.; Lin, W.; Maier, A.; Fiebig, H.H.; Hertweck, C. Bacaryolanes A–C, rare bacterial caryolanes from a mangrove endophyte. J. Nat. Prod. 2015, 78, 2963–2967. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, Y.; Huang, G.D.; Wu, W.S. Four new compounds from Sinacalia tangutica. Helv. Chim. Acta 2008, 91, 1894–1901. [Google Scholar] [CrossRef]
- Brady, S.F.; Singh, M.P.; Janso, J.E.; Clardy, J. Guanacastepene, a fungal-derived diterpene antibiotic with a new carbon skeleton. J. Am. Chem. Soc. 2000, 122, 2116–2117. [Google Scholar] [CrossRef]
- Brady, S.F.; Bondi, S.M.; Clardy, J. The guanacastepenes: A highly diverse family of secondary metabolites produced by an endophytic fungus. J. Am. Chem. Soc. 2001, 123, 9900–9901. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Janso, J.E.; Luckman, S.W.; Brady, S.F.; Clardy, J.; Greenstein, M.; Maiese, W.M. Biological activity of guanacastepene, a novel diterpenoid antibiotic produced by an unidentified fungus CR115. J. Antibiot. 2000, 53, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.K.; Hughes, C.C.; Kennedy-Smith, J.J.; Gradl, S.N.; Trauner, D. Total synthesis of (−)-heptemerone B and (−)-guanacastepene E. J. Am. Chem. Soc. 2006, 128, 17057–17062. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Z.; Sha, C.K. Stereoselective total syntheses of guanacastepenes N and O. Org. Lett. 2015, 17, 3486–3489. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.P.; Liang, X.R.; Yin, X.L.; Wang, G.; Ji, N.Y. Absolute configurations of unique harziane diterpenes from Trichoderma species. Org. Lett. 2012, 14, 3815–3817. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, H.; Li, H.; Sun, X.; Huang, H.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B from the culture of Aspergillus sp. 16-5c. Org. Lett. 2013, 15, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Y.; Chen, S.; Liu, Y.; Lu, Y.; Chen, D.; Lin, Y.; Huang, X.; She, Z. Aspterpenacids A and B, two sesterterpenoids from a mangrove endophytic fungus Aspergillus terreus H010. Org. Lett. 2016, 18, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.E.; Huang, H.R.; Shao, C.L.; Xia, X.K.; Ma, L.; Huang, X.S.; Lu, Y.J.; Lin, Y.C.; Long, Y.H.; She, Z.G. Asperterpenols A and B, new sesterterpenoids isolated from a mangrove endophytic fungus Aspergillus sp. 085242. Org. Lett. 2013, 15, 2522–2525. [Google Scholar] [CrossRef] [PubMed]
- Skellam, E. The biosynthesis of cytochalasans. Nat. Prod. Rep. 2017, 34, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tao, X.; Liu, J.; Chen, R.; Zhang, M.; Li, L.; Fang, X.; Yu, L.-Y.; Dai, J. Periconiasin G, a new cytochalasan with unprecedented 7/6/5 tricyclic ring system from the endophytic fungus Periconia sp. Tetrahedron Lett. 2016, 57, 796–799. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Zhang, M.; Liu, X.; Chen, R.; Zhao, J.; Li, L.; Wang, N.; Dai, J. Periconiasins I and J, two new cytochalasans from an endophytic fungus Periconia sp. Tetrahedron Lett. 2016, 57, 5794–5797. [Google Scholar] [CrossRef]
- Zhang, D.; Tao, X.; Chen, R.; Liu, J.; Li, L.; Fang, X.; Yu, L.; Dai, J. Pericoannosin A, a polyketide synthase-nonribosomal peptide synthetase hybrid metabolite with new carbon skeleton from the endophytic fungus Periconia sp. Org. Lett. 2015, 17, 4304–4307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Ge, H.L.; Xie, D.; Chen, R.D.; Zou, J.H.; Tao, X.Y.; Dai, J.G. Periconiasins A–C, new cytotoxic cytochalasans with an unprecedented 9/6/5 tricyclic ring system from endophytic fungus Periconia sp. Org. Lett. 2013, 15, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-W.; Tao, X.-Y.; Liu, J.-M.; Chen, R.-D.; Zhang, M.; Fang, X.-M.; Yu, L.-Y.; Dai, J.-G. A new polyketide synthase−nonribosomal peptide synthetase hybrid metabolite from plant endophytic fungus Periconia sp. Chin. Chem. Lett. 2016, 27, 640–642. [Google Scholar] [CrossRef]
- Tian, C.; Lei, X.; Wang, Y.; Dong, Z.; Liu, G.; Tang, Y. Total syntheses of periconiasins A–E. Angew. Chem. Int. Ed. 2016, 55, 6992–6996. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wang, H.; Li, L.; Song, B.; Chen, H.; Zhang, H.; Liu, X.; Zou, Z. Trichodermone, a spiro-cytochalasan with a tetracyclic nucleus (7/5/6/5) skeleton from the plant endophytic fungus Trichoderma gamsii. J. Nat. Prod. 2014, 77, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Wang, H.; Li, L.; Chen, A.J.; Chen, L.; Chen, H.; Zhang, H.; Liu, X.; Zou, Z. Trichoderones A and B: Two pentacyclic cytochalasans from the plant endophytic fungus Trichoderma gamsii. Eur. J. Org. Chem. 2012, 2012, 2516–2519. [Google Scholar] [CrossRef]
- Yan, B.C.; Wang, W.G.; Hu, D.B.; Sun, X.; Kong, L.M.; Li, X.N.; Du, X.; Luo, S.H.; Liu, Y.; Li, Y.; et al. Phomopchalasins A and B, two cytochalasans with polycyclic-fused skeletons from the endophytic fungus Phomopsis sp. shj2. Org. Lett. 2016, 18, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, X.; Feng, H.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Campyridones A–D, pyridone alkaloids from a mangrove endophytic fungus Campylocarpon sp. HDN13-307. Tetrahedron 2016, 72, 5679–5683. [Google Scholar] [CrossRef]
- Maloney, K.N.; Hao, W.; Xu, J.; Gibbons, J.; Hucul, J.; Roll, D.; Brady, S.F.; Schroeder, F.C.; Clardy, J. Phaeosphaeride A, an inhibitor of STAT3-dependent signaling isolated from an endophytic fungus. Org. Lett. 2006, 8, 4067–4070. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Okamoto, I.; Morita, N.; Kiyotani, T.; Tamura, O. Synthesis of the proposed structure of phaeosphaeride A. Org. Biomol. Chem. 2011, 9, 5825–5832. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kobayashi, Y.; Nakamura, M.; Tamura, O.; Kogen, H. Establishment of relative and absolute configurations of phaeosphaeride A: Total synthesis of ent-phaeosphaeride A. J. Org. Chem. 2015, 80, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Abzianidze, V.V.; Poluektova, E.V.; Bolshakova, K.P.; Panikorovskii, T.L.; Bogachenkov, A.S.; Berestetskiy, A.O. Crystal structure of natural phaeosphaeride A. Acta Crystallogr. Sect. E 2015, 71 Pt 8, o625–o626. [Google Scholar] [CrossRef] [PubMed]
- Chatzimpaloglou, A.; Yavropoulou, M.P.; Rooij, K.E.; Biedermann, R.; Mueller, U.; Kaskel, S.; Sarli, V. Total synthesis and biological activity of the proposed structure of phaeosphaeride A. J. Org. Chem. 2012, 77, 9659–9667. [Google Scholar] [CrossRef] [PubMed]
- Abzianidze, V.V.; Prokofieva, D.S.; Chisty, L.A.; Bolshakova, K.P.; Berestetskiy, A.O.; Panikorovskii, T.L.; Bogachenkov, A.S.; Holder, A.A. Synthesis of natural phaeosphaeride A derivatives and an in vitro evaluation of their anti-cancer potential. Bioorg. Med. Chem. Lett. 2015, 25, 5566–5569. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Ding, Y.; Yang, B.J.; Miklossy, G.; Yin, H.Q.; Walker, L.A.; Turkson, J.; Cao, S. A new metabolite with a unique 4-pyranone-gamma-lactam-1,4-thiazine moiety from a Hawaiian-plant associated fungus. Org. Lett. 2015, 17, 3556–3559. [Google Scholar] [CrossRef] [PubMed]
- Trenti, F.; Cox, R.J. Structural revision and biosynthesis of the fungal phytotoxins phyllostictines A and B. J. Nat. Prod. 2017, 80, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.F.; Kurtan, T.; Yang, X.H.; Mandi, A.; Geng, M.Y.; Ye, B.P.; Taglialatela-Scafati, O.; Guo, Y.W. Penibruguieramine A, a novel pyrrolizidine alkaloid from the endophytic fungus Penicillium sp. GD6 associated with Chinese mangrove Bruguiera gymnorrhiza. Org. Lett. 2014, 16, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.; Kim, S. Biomimetic total synthesis of (−)-penibruguieramine A using memory of chirality and dynamic kinetic resolution. Angew. Chem. Int. Ed. 2015, 54, 10875–10878. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Strobel, G.; Harper, J.; Lobkovsky, E.; Clardy, J. Cryptocin, a potent tetramic acid antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Org. Lett. 2000, 2, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kusari, S.; Spiteller, M. Natural products containing ‘decalin’ motif in microorganisms. Nat. Prod. Rep. 2014, 31, 1175–1201. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Rao, M.; Ou, J.; Yin, J.; Lu, W.; Liu, M.; Pang, X.; Gao, S. Total synthesis and biological studies of cryptocin and derivatives of equisetin and fusarisetin A. Org. Biomol. Chem. 2014, 12, 7591–7597. [Google Scholar] [CrossRef] [PubMed]
- Kontnik, R.; Clardy, J. Codinaeopsin, an antimalarial fungal polyketide. Org. Lett. 2008, 10, 4149–4151. [Google Scholar] [CrossRef] [PubMed]
- Ondeyka, J.G.; Helms, G.L.; Hensens, O.D.; Goetz, M.A.; Zink, D.L.; Tsipouras, A.; Shoop, W.L.; Slayton, L.; Dombrowski, A.W.; Polishook, J.D.; et al. Nodulisporic acid A, a novel and potent insecticide from a Nodulisporium sp. isolation, structure determination, and chemical transformations. J. Am. Chem. Soc. 1997, 119, 8809–8816. [Google Scholar] [CrossRef]
- Gao, S.S.; Li, X.M.; Williams, K.; Proksch, P.; Ji, N.Y.; Wang, B.G. Rhizovarins A–F, indole-diterpenes from the mangrove-derived endophytic fungus Mucor irregularis QEN-189. J. Nat. Prod. 2016, 79, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.Y.; Liu, X.H.; Miao, F.P.; Qiao, M.F. Aspeverin, a new alkaloid from an algicolous strain of Aspergillus versicolor. Org. Lett. 2013, 15, 2327–2329. [Google Scholar] [CrossRef] [PubMed]
- Mugishima, T.; Tsuda, M.; Kasai, Y.; Ishiyama, H.; Fukushi, E.; Kawabata, J.; Watanabe, M.; Akao, K.; Kobayashi, J. Absolute stereochemistry of citrinadins A and B from marine-derived fungus. J. Org. Chem. 2005, 70, 9430–9435. [Google Scholar] [CrossRef] [PubMed]
- Finefield, J.M.; Frisvad, J.C.; Sherman, D.H.; Williams, R.M. Fungal origins of the bicyclo[2.2.2]diazaoctane ring system of prenylated indole alkaloids. J. Nat. Prod. 2012, 75, 812–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levinson, A.M. Total synthesis of aspeverin via an iodine(III)-mediated oxidative cyclization. Org. Lett. 2014, 16, 4904–4907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Mandi, A.; Li, X.M.; Du, F.Y.; Wang, J.N.; Li, X.; Kurtan, T.; Wang, B.G. Varioxepine A, a 3H-oxepine-containing alkaloid with a new oxa-cage from the marine algal-derived endophytic fungus Paecilomyces variotii. Org. Lett. 2014, 16, 4834–4837. [Google Scholar] [CrossRef] [PubMed]
- Doveston, R.G.; Taylor, R.J.K. An expedient synthesis of the proposed biosynthetic precursor of the oxepine natural product, janoxepin. Tetrahedron Lett. 2012, 53, 2533–2536. [Google Scholar] [CrossRef]
- Hemberger, Y.; Xu, J.; Wray, V.; Proksch, P.; Wu, J.; Bringmann, G. Pestalotiopens A and B: Stereochemically challenging flexible sesquiterpene-cyclopaldic acid hybrids from Pestalotiopsis sp. Chem. Eur. J. 2013, 19, 15556–15564. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Ren, G.; Yang, B.J.; Miklossy, G.; Turkson, J.; Fei, P.; Ding, Y.; Walker, L.A.; Cao, S. Meroterpenoids with antiproliferative activity from a Hawaiian-plant associated fungus Peyronellaea coffeae-arabicae FT238. Org. Lett. 2016, 18, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, J.; Praseuth, M.B.; Tyan, S.W.; Sanchez, J.F.; Entwistle, R.; Chiang, Y.M.; Oakley, B.R.; Wang, C.C. Molecular genetic characterization of the biosynthesis cluster of a prenylated isoindolinone alkaloid aspernidine A in Aspergillus nidulans. Org. Lett. 2013, 15, 2862–2865. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, X.; Li, N.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Isoindolone-containing meroperpenoids from the endophytic fungus Emericella nidulans HDN12-249. Org. Lett. 2016, 18, 4670–4673. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, C.W. The isolation and structure of cyclopiazonic acid, a toxic metabolite of Penicillium cyclopium Westling. Tetrahedron 1968, 24, 2101–2119. [Google Scholar] [CrossRef]
- Zhou, M.; Miao, M.M.; Du, G.; Li, X.N.; Shang, S.Z.; Zhao, W.; Liu, Z.H.; Yang, G.Y.; Che, C.T.; Hu, Q.F.; et al. Aspergillines A–E, highly oxygenated hexacyclic indole-tetrahydrofuran-tetramic acid derivatives from Aspergillus versicolor. Org. Lett. 2014, 16, 5016–5019. [Google Scholar] [CrossRef] [PubMed]
- Seshime, Y.; Juvvadi, P.R.; Tokuoka, M.; Koyama, Y.; Kitamoto, K.; Ebizuka, Y.; Fujii, I. Functional expression of the Aspergillus flavus PKS-NRPS hybrid CpaA involved in the biosynthesis of cyclopiazonic acid. Bioorg. Med. Chem. Lett. 2009, 19, 3288–3292. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Walsh, C.T. Cyclopiazonic acid biosynthesis in Aspergillus sp.: Characterization of a reductase-like R* domain in cyclopiazonate synthetase that forms and releases cyclo-acetoacetyl-l-tryptophan. Biochemistry 2009, 48, 8746–8757. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Walsh, C.T. Characterization of cyclo-acetoacetyl-L-tryptophan dimethylallyltransferase in cyclopiazonic acid biosynthesis: Substrate promiscuity and site directed mutagenesis studies. Biochemistry 2009, 48, 11032–11044. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





























| Microorganisms | Origin | Secondary Metabolites (Figures) | Reported Activities a | Ref. |
|---|---|---|---|---|
| Streptomyces sp. | Marine, Bruguiera gymnorrhiza | Divergolides A–D (1–4) (Figure 1) | Antibacterial, cytotoxicity | [28] |
| Streptomyces sp. | Terrestrial, Camellia sinensis | Rubrolone B (43) (Figure 7) | NR b | [61] |
| Streptomyces sp. | Marine, Bruguiera gymnorrhiza | Bacaryolanes A–C (81–83) (Figure 13) | NR | [108] |
| Chalara sp. | Terrestrial, Artemisia vulgaris | Isofusidienols A–D (22–25) (Figure 4) | Antibacterial | [39] |
| A nonsporulating fungus | Terrestrial, Knightia excelsa | Spiro-mamakone A (32) (Figure 5) | Antibacterial, cytotoxicity | [45] |
| Penicillium brocae | Marine | Spirobrocazines A–B (57–58) (Figure 10) | Antibacterial, cytotoxicity | [83] |
| Pestalotiopsis fici | Terrestrial | Chloropupukeananin (72) (Figure 13) | Antibacterial, antiviral | [100] |
| Unidentified fungus | Terrestrial, Daphnopsis americana | Guanacastepene A (84) (Figure 14) | Antibacterial | [110,111] |
| Blennoria sp. | Terrestrial, Carpobrotus edulis | Blennolides A–G (11–17) (Figure 3) | Antifungal, antialgal | [36] |
| Cryptosporiopsis sp. | Terrestrial, Viburnum tinus | Viburspiran (48) (Figure 8) | Antifungal | [74] |
| Cryptosporiopsis cf. quercina | Terrestrial, Triptergyium wilfordii | Cryptocin (110) (Figure 18) | Antifungal | [140] |
| Cephalosporium acremonium | Terrestrial, Trachelospermum jasminoides | Cephalosol (45) (Figure 8) | Antimicrobial | [68] |
| Daldinia eschscholtzii | Terrestrial, Paphiopedilum exul | Daldionin (47) (Figure 8) | Antimicrobial | [73] |
| Rhizoctonia solani | Terrestrial, Cyperus rotundus | Solanioic acid (65) (Figure 12) | Antimicrobial | [94] |
| Trichoderma spp. | Marine, alga Codium fragile | Harziandione (85) and harzianone (86) (Figure 14) | Antimicrobial | [115] |
| Paecilomyces variotii | Marine | Varioxepine A (116) (Figure 19) | Antimicrobial | [150] |
| Pestalotiopsis sp. | Marine, Rhizophora mucronata | Pestalotiopens A–B (117–118) (Figure 20) | Antimicrobial | [152] |
| Pestalotiopsis fici | Terrestrial | Chloropestolide A (73) (Figure 13) | Anti-HIV, cytotoxicity | [102] |
| Periconia sp. | Terrestrial, Annona muricata | Pericoannosin A (96) (Figure 16) | Anti-HIV | [122] |
| Neosartorya udagawae | Marine, Aricennia marina | Neosartoryadins A–B (59–60) (Figure 11) | Antiviral | [84] |
| Periconia sp. | Terrestrial, Annona muricata | periconiasins (92–95) (Figure 16) | Antiviral, cytotoxicity | [120,121,123] |
| Aspergillus versicolor | Terrestrial, polyphylla var. yunnanensis | Aspergillines A–E (125–129) (Figure 22) | Antiviral, cytotoxicity | [157] |
| Periconia sp. | Terrestrial, Annonsa muricata | Periconianone A (79) (Figure 13) | Anti-inflammatory | [105] |
| Unidentified fungus | Terrestrial, Vochysia guatemalensis | Codinaeopsin (111) (Figure 18) | Antimalarial | [143] |
| Phomopsis sp. | Terrestrial, Isodon eriocalyx var. laxiflora | Phomopchalasins A–B (100–101) (Figure 17) | Antimigratory activity | [128] |
| Fusarium pallidoroseum | Terrestrial | Apicidins A–C (62–64) (Figure 11) | Antiprotozoal, anticancer | [90] |
| Actinoallomurus fulvus | Terrestrial, Capsicum frutescens | Actinoallolides A–E (5–9) (Figure 2) | Anti-trypanosomal | [34] |
| Aspergillus sp. | Marine | Asperterpenoid A (87) (Figure 15) | Antituberculosis | [116] |
| Aspergillus sp. | Marine | Asperterpenols A–B (88–89) (Figure 15) | Acetylcholinesterase inhibition | [118] |
| Cytospora sp. | Terrestrial, Conocarpus erecta | Cytoskyrins A–B (52–53) (Figure 9) | BIA activity | [78] |
| Unidentified fungus | Terrestrial, Ficus microcarpa L. | Microcarpalide (10) (Figure 2) | Cytotoxicity | [35] |
| Nodulisporium sp. | Marine, Alga | Noduliprevenone (18) (Figure 3) | Cytotoxicity | [37] |
| Pestalotiopsis virgatula | Terrestrial, Dracontomelon duperreanum | Virgatolides A–C (27–29) (Figure 5) | Cytotoxicity | [41] |
| Pestalotiopsis microspora | Terrestrial, Torreya taxifolia | (±)-torreyanic acid (36) (Figure 6) | Cytotoxicity | [50] |
| Chaetomium globosum | Terrestrial, Imperata cylindrical | Chaetoglobins A–B (38–39) (Figure 7) | Cytotoxicity | [56] |
| Alternaria sp. | Terrestrial, Carex aridula | (-)-Alternarlactam (40) (Figure 7) | Cytotoxicity | [57] |
| Penicillium manginii | Terrestrial, Panax notoginseng | Duclauxamide A1 (42) (Figure 7) | Cytotoxicity | [59] |
| Penicillium sp. | Terrestrial, Catharanthus roseus | Citreoviripyrone A (46) (Figure 8) | Cytotoxicity | [71] |
| Chaetomium sp. | Terrestrial, Adenophora axilliflora | Chaetominine (61) (Figure 11) | Cytotoxicity | [86] |
| Pestalotiopsis fici | Terrestrial | Chloropupukeanolides A–E (74–78) (Figure 13) | Cytotoxicity | [103,104] |
| Trichoderma gamsii | Terrestrial, Panax notoginseng | Trichoderones A–B (97–98) (Figure 16) | Cytotoxicity | [127] |
| Trichoderma gamsii | Terrestrial, Panax notoginseng | Trichodermone (99) (Figure 16) | Cytotoxicity | [126] |
| Campylocarpon sp. | Marine, Sonneratia caseolaris | Campyridones A–D (102–105) (Figure 17) | Cytotoxicity | [129] |
| Mucor irregularis | Marine, Rhizophora stylosa | Rhizovarins A–C (112–114) (Figure 19) | Cytotoxicity | [145] |
| Peyronellaea coffeae-arabicae | Terrestrial, Pritchardia lowreyana | Peyronellins A–C (119–121) (Figure 20) | Cytotoxicity | [153] |
| Pestalotiopsis sp. | Terrestrial, Taxus brevifolia | Pestalotiopsin A (80) (Figure 13) | Immunosuppressive | [107] |
| Penicillium dangeardii | Terrestrial, Lysidice rhodostegia | Penicillactones A–C (33–35) (Figure 5) | Inhibitors of the release of β-glucuronidase | [48] |
| Phaeosphaeria avenaria | Terrestrial | Phaeosphaeride A–B (106–107) (Figure 18) | Inhibiting STAT3 activity | [130] |
| Aspergillus versicolor | Marine, green alga Codium fragile | Aspeverin (115) (Figure 19) | Marine plant growth inhibition | [146] |
| Microsphaeropsis sp. | Terrestrial, Lycium intricatum | Microsphaeropsones A–C (19–21) (Figure 4) | NR | [38] |
| Paraphaeosphaeria neglecta | Terrestrial, Lycopodiella cernua | Lycopodiellactone (26) (Figure 4) | NR | [40] |
| Pestalotiopsis virgatula | Terrestrial, Terminalia chebula | Pestalospiranes A–B (30–31) (Figure 5) | NR | [43] |
| Pestalotiopsis sp. | Terrestrial, Clavaroids sp. | Torreyanic acid analogue (37) (Figure 6) | NR | [53] |
| Fusarium sp. | Terrestrial, Melia azedarach | Fusarimine (41) (Figure 7) | NR | [58] |
| Fusidium sp. | Terrestrial, Mentha arvensis | Fusidilactone C (44) (Figure 8) | NR | [65,66] |
| Corynespora cassiicola | Marine, Laguncularia racemosa | Coryoctalactone E (49) (Figure 8) | NR | [75] |
| Colletotrichum capsici | Terrestrial, Siegesbeckia pubescens | Citrinals A–B (50–51) (Figure 9) | NR | [76,77] |
| Aspergillus sp. | Terrestrial, Melia azedarach L. | Aspertryptanthrins A–C (54–56) (Figure 10) | NR | [82] |
| Chalara alabamensis | Terrestrial, Asterogyne martiana | Asterogynins A–B (66–67) (Figure 12) | NR | [96] |
| Talaromyces wortmannii | Terrestrial, Tripterygium wilfordii | Wortmannines A–C (68–70) (Figure 12) | NR | [98] |
| Talaromyces wortmanni | Terrestrial, Tripterygium wilfordii | Secovironolide (71) (Figure 12) | NR | [97] |
| Aspergillus sp. | Marine, Kandelia obovata | Aspterpenacids A–B (90–91) (Figure 15) | NR | [117] |
| Paraphaeosphaeria neglecta | Terrestrial, Lycopodiella cernua | Paraphaeosphaeride A (108) (Figure 18) | NR | [136] |
| Penicillium sp. | Marine, Bruguiera gymnorrhiza | Penibruguieramine A (109) (Figure 18) | NR | [138] |
| Emericella nidulans | Terrestrial, Tamarix chinensis Lour | Emericellolides A–C (122–124) (Figure 21) | NR | [155] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Li, G.; Lou, H.-X. Structural Diversity and Biological Activities of Novel Secondary Metabolites from Endophytes. Molecules 2018, 23, 646. https://doi.org/10.3390/molecules23030646
Gao H, Li G, Lou H-X. Structural Diversity and Biological Activities of Novel Secondary Metabolites from Endophytes. Molecules. 2018; 23(3):646. https://doi.org/10.3390/molecules23030646
Chicago/Turabian StyleGao, Han, Gang Li, and Hong-Xiang Lou. 2018. "Structural Diversity and Biological Activities of Novel Secondary Metabolites from Endophytes" Molecules 23, no. 3: 646. https://doi.org/10.3390/molecules23030646