Uses of Cyanoacetylhydrazine in Heterocyclic Synthesis: Novel Synthesis of Pyrazole Derivatives with Anti-tumor Activities
Abstract
:1. Introduction
2. Results and Discussion
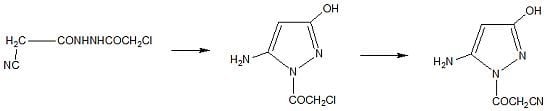
2.1. Chemistry



2.2. Antitumor Evaluations
2.2.1. Structure Activity Relationship of the Newly Synthesized Products
| Compound | GI50 (mol L−1) | ||
|---|---|---|---|
| MCF-7 | NCI-H460 | SF-268 | |
| 3 | 20.1 ± 0.6 | 16.3 ± 1.4 | 22.3 ± 1.5 |
| 4 | 22.6 ± 0.4 | 21.3 ± 0.8 | 22 ± 0.8 |
| 5 | 40.6 ± 16.9 | 38.9 ± 10.8 | 20.8 ± 8.6 |
| 7a | 40.6 ± 12.2 | 32.6 ± 8.6 | 60.4 ± 14.8 |
| 7b | 35.4 ± 8.2 | 26.1 ± 2.8 | 28.9 ± 4.8 |
| 8 | 11.8 ± 0.6 | 14.5 ± 0.8 | 16.7 ± 1.6 |
| 10 | 33.7 ± 17.5 | 20.2 ± 8.8 | 12.0 ± 2.4 |
| 11a | 2.1 ± 0.7 | 1.2 ± 0.8 | 1.4 ± 0.8 |
| 11b | 20.0 ± 1.2 | 20.6 ± 3.4 | 18.4 ± 2.6 |
| 12a | 0.01 ± 0.001 | 0.01 ± 0.008 | 0.02 ± 0.001 |
| 12b | 16.0 ± 3.6 | 20.0 ± 2.4 | 18.5 ± 6.0 |
| 14 | 50.6 ± 12.9 | 36.4 ± 8.8 | 44.8 ± 6.6 |
| 17a | 0.1± 0.02 | 0.4 ± 0.01 | 0.4 ± 0.08 |
| 17b | 12.4 ± 8.2 | 10.1 ± 2.8 | 8.2 ± 1.8 |
| 17c | 6.2 ± 1.6 | 4.2 ± 1.8 | 2.7 ± 0.6 |
| 17d | 0.2 ± 0.01 | 0.1 ± 0.06 | 0.3 ± 0.05 |
| 18a | 0.02 ± 0.008 | 0.03 ± 0.008 | 0.01 ± 0.004 |
| 18b | 20.0 ± 3.6 | 22.0 ± 2.4 | 31.5 ± 8.0 |
| 18c | 0.03 ± 0.006 | 0.01 ± 0.006 | 0.03 ± 0.005 |
| 18d | 1.9 ± 0.9 | 0.6 ± 1.8 | 0.8 ± 0.08 |
| Doxorubicin | 0.04 ± 0.008 | 0.09 ± 0.008 | 0.09 ± 0.007 |
3. Experimental
3.1. Antitumor Activity Tests
3.2. Chemistry
4. Conclusions
Acknowledgements
References
- Liu, X.H.; Cui, P.; Song, B.A.; Bhadury, P.S.; Zhu, H.L.; Wang, S.F. Synthesis, structure and antibacterial activity of novel 1-(5-substituted-3-substituted-4,5-dihydropyrazol-1-yl)ethanone oxime ester derivatives. Bioorgan. Med. Chem. 2008, 16, 4075–4082. [Google Scholar]
- Ouyang, G.; Chen, Z.; Cai, X.J.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activity of novel pyrazole derivatives containing oxime esters group. Bioorgan. Med. Chem. 2008, 16, 9699–9707. [Google Scholar] [CrossRef]
- Abdel-Hafez, E.M.N.; Rahma, G.E.A.A.; Aziz, M.A.; Radwan, M.F.; Farag, H.H. Design, synthesis and biological investigation of certain pyrazole-3-carboxylic acid derivatives as novel carriers for nitric oxide. Bioorgan. Med. Chem. 2009, 17, 3829–3837. [Google Scholar]
- Park, H.J.; Lee, K.; Park, S.J.; Ahn, B.; Lee, J.C.; Cho, H.Y.; Lee, K.I. Identification of antitumor activity of pyrazole oxime ethers. Bioorgan. Med. Chem. Lett. 2005, 15, 3307–3312. [Google Scholar]
- Ouyang, G.; Cai, X.J.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J. Agric. Food Chem. 2008, 56, 101601–102607. [Google Scholar]
- Dai, H.; Li, Y.Q.; Du, D.; Qin, X.; Zhang, X.; Yu, H.B.; Fang, J.X. Synthesis and biological activities of novel pyrazole oxime derivatives containing a 2-chloro-5-thiazolyl moiety. Food Chem. 2008, 56, 10805–10810. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Farghaly, T.A.; Abdallah, M.A.; Abdalla, M.M.; El-Aziz, M.R.A. New pyrazoles incorporating pyrazolylpyrazole moiety: Synthesis, anti-HCV and antitumor activity. Eur. J. Med. Chem. 2010, 45, 1042–1050. [Google Scholar]
- Anzaldi, M.; Maccio, C.; Mazzei, M.; Bertolotto, M.; Ottonello, L.; Dallegri, F.; Balbi, A. Antiproliferative and proapoptotic activities of a new class of pyrazole derivatives in HL-60 cells. Chem. Biodivers. 2009, 6, 1674–1684. [Google Scholar]
- El-Shafei, A.; Fadda, A.A.; Khalil, A.M.; Ameen, T.A.E.; Badria, F.A. Synthesis, antitumor evaluation, molecular modeling and quantitative structure-activity relationship (QSAR) of some novel arylazopyrazolodiazine and triazine analogs. Bioorgan. Med. Chem. 2009, 17, 5096–5105. [Google Scholar]
- Huang, K.H.; Veal, J.M.; Fadden, P.R.; Rice, J.W.; Eaves, J.; Strachan, J.P.; Barabasz, A.F.; Foley, B.E.; Barta, T.E.; Ma, W.; et al. Discovery of Novel 2-Aminobenzamide Inhibitors of Heat Shock Protein 90 as Potent, Selective and Orally Active Antitumor Agents. J. Med. Chem. 2009, 52, 4288–4305. [Google Scholar]
- Chandanshive, J.Z.; Bonini, B.F.; Tiznado, W.; Escobar, C.A.; Caballero, J.; Femoni, C.; Fochi, M.; Franchini, M.C. 1,3-Dipolar Cycloaddition of Nitrile Imines with Cyclic α-β-Unsaturated Ketones: A Regiochemical Route to Ring-Fused Pyrazoles. Eur. J. Org. Chem. 2011, 4806–4811. [Google Scholar]
- Chandanshive, J.Z.; Bonini, B.F.; Gentili, D.; Fochi, M.; Bernardi, L.; Franchini, M.C. Regiocontrolled Synthesis of Ring-Fused Thieno[2,3-c]pyrazoles through 1,3-Dipolar Cycloaddition of Nitrile Imines with Sulfur-Based Acetylenes. Eur. J. Org. Chem. 2010, 33, 6440–6447. [Google Scholar]
- Bondock, S.; Fadaly, W.; Metwally, M.A. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur. J. Med. Chem. 2010, 45, 3692–3701. [Google Scholar]
- Mohareb, R.M.; Samir, E.M. The Reaction of cyanoacetylhydrazine with chloroacetone: Synthesis of 1,2,4-triazine, 1,3,4–oxadiazine and their fused derivatives with antitumor activities. Open J. Med. Chem. 2012, 2, 1–9. [Google Scholar] [CrossRef]
- Mohareb, R.M.; Ibrahim, R.A.; Moustafa, H.E. Hydrazide-hydrazones in the synthesis of 1,3,4-oxadiazine, 1,2,4-triazine and pyrazole derivatives with anti-tumor activities. Open Org. Chem. J. 2010, 4, 8–14. [Google Scholar]
- Mohareb, R.M.; Schatz, J. Anti-tumor and anti-leishmanial evaluations of 1,3,4-oxadiazine, pyran derivatives derived from cross-coupling reactions of β-bromo-6H-1,3,4- oxadiazine derivatives. Bioorgan. Med. Chem. 2011, 19, 2707–2713. [Google Scholar] [CrossRef]
- Khalil, A.M.; Berghot, M.A.; Gouda, M.A. Synthesis and antibacterial activity of some new thiazole and thiophene derivatives. Eur. J. Med. Chem. 2009, 44, 4434–4440. [Google Scholar]
- McKibben, B.P.; Cartwright, C.H.; Castelhano, A.L. Practical synthesis of tetrasubstituted thiophenes for use in compound libraies. Tetrahedron 1999, 40, 5471–5474. [Google Scholar] [CrossRef]
- Balamurugan, K.; Perumal, S.; Sunil, A.; Reddy, A.S.K.; Yogeeswari, P.; Sriram, D.A. facile domino protocol for the regioselective synthesis and discovery of novel 2-amino-5-arylthieno [2,3-b] thiophene as antimycobacterial agents. Tetrahedron Lett. 2009, 50, 6191–6195. [Google Scholar]
- Ye, D.; Zhang, Y.; Wang, F.; Zheng, M.; Zhang, X.; Luo, X.; Shen, X.; Jiang, H.; Liu, H. Novel thiophene derivatives as PTP1B inhibitors with selectivity and cellular activity. Bioorgan. Med. Chem. 2010, 18, 1773–1782. [Google Scholar]
- Mansour, S.A.; Mahmoud, S.B.; Saleh, I.A.; Mostafa, M.G. Ant-breast cancer activity of some novel 1,2-dihydropyridine, thiophene and thiazole derivatives. Eur. J. Med. Chem. 2011, 46, 137–141. [Google Scholar] [CrossRef]
- Scrowston, R.M. Recent advances in the chemistry of benzo [b]-thiophene. Adv. Heterocycl. Chem. 1981, 29, 171–249. [Google Scholar] [CrossRef]
- Parida, K.M.; Rath, D. Amine functionalized MCM-41: An active and reusable catalyst for Knoevenagel condensation reaction. J. Mol. Catal. A: Chem. 2009, 310, 93–100. [Google Scholar] [CrossRef]
- Jain, D.; Mishra, M.; Rani, A. Synthesis and characterization of novel aminopropylated fly ash catalyst and its beneficial application in base catalyzed Knoevenagel condensation reaction. Fuel Process. Technol. 2012, 95, 119–126. [Google Scholar] [CrossRef]
- Tisseh, Z.N.; Dabiri, M.; Nobahar, M.; Khavasi, H.R.; Bazgir, A. Catalyst-free, aqueous and highly diastereoselective synthesis of new 5-substituted 1H-tetrazoles via a muti-component domino Knoevenagel condensation/1,3- dipolar cycloaddition reaction. Tetrahedron 2012, 68, 1769–1773. [Google Scholar]
- Atamanyuk, D.V.; Ostapiuk, Y.V.; Kryshchyshyn, A.P. A new domino-Knoevenagel-hetero-Diels-Alder reaction. Tetrahedron Lett. 2008, 49, 4648–4651. [Google Scholar]
- Cateni, F.; Zilic, J.; Zacchigna, M.; Bonivento, P.; Frausin, F.; Scarcia, V. Synthesis and biological properties of new α-methylene-butyrolactones and α,β-unsaturated γ-butyrolactones. Eur. J. Med. Chem. 2006, 41, 192–200. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenne, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paul, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar]
- Sample Availability: Samples of compounds 3, 5, 7a,b, 8, 10, 11a,b, 12a,b, 14, 17a–d and 18a–d are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mohareb, R.M.; El-Sayed, N.N.E.; Abdelaziz, M.A. Uses of Cyanoacetylhydrazine in Heterocyclic Synthesis: Novel Synthesis of Pyrazole Derivatives with Anti-tumor Activities. Molecules 2012, 17, 8449-8463. https://doi.org/10.3390/molecules17078449
Mohareb RM, El-Sayed NNE, Abdelaziz MA. Uses of Cyanoacetylhydrazine in Heterocyclic Synthesis: Novel Synthesis of Pyrazole Derivatives with Anti-tumor Activities. Molecules. 2012; 17(7):8449-8463. https://doi.org/10.3390/molecules17078449
Chicago/Turabian StyleMohareb, Rafat M., Nahed N. E. El-Sayed, and Mahmoud A. Abdelaziz. 2012. "Uses of Cyanoacetylhydrazine in Heterocyclic Synthesis: Novel Synthesis of Pyrazole Derivatives with Anti-tumor Activities" Molecules 17, no. 7: 8449-8463. https://doi.org/10.3390/molecules17078449