Micro-Raman Study of Thermal Transformations of Sulfide and Oxysalt Minerals Based on the Heat Induced by Laser
Abstract
:1. Introduction
2. Samples and Methods
2.1. Samples Characteristics
2.2. Methods
3. Results and Discussion
3.1. Thermal Transformations on the Sulfide Minerals
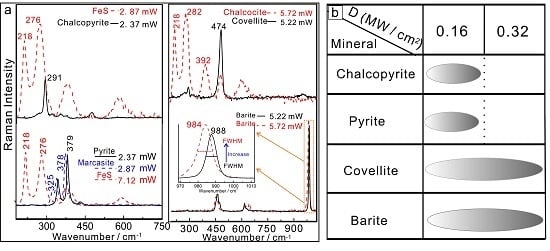
3.1.1. Thermal Transformations of High-Temperature Chalcopyrite
3.1.2. Thermal Transformations of Moderate- to Low-Temperature Covellite
3.1.3. Thermal Transformations of Moderate- to Low-Temperature Pyrite
3.1.4. Possible Explanations for the Thermal Transformations of Sulfide Minerals
3.2. Thermal Transformations Study of the Oxysalt Mineral
3.2.1. Thermal Transformations of the Low-Temperature Sulfate Mineral Barite
3.2.2. No Thermal Transformations of the Low-Temperature Carbonate Mineral Aragonite
3.3. Minimum Laser Power Densities for Thermal Transformations of Minerals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weiss, R.F.; Lonsdale, P.; Lupton, J.E.; Bainbridge, A.E.; Craig, H. Hydrothermal plumes in the Galapagos Rift. Nature 1977, 267, 600–603. [Google Scholar] [CrossRef]
- Rona, P.A.; Lowell, R.P. Hydrothermal Systems at Oceanic Spreading Centers. Geology 1978, 6, 299–300. [Google Scholar] [CrossRef]
- Jannasch, H.W.; Mottl, M.J. Geomicrobiology of deep-sea hydrothermal vents. Science 1985, 229, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Lilley, M.D.; Butterfield, D.A.; Olson, E.J.; Lupton, J.E.; Macko, S.A.; Mcduff, R.E. Anomalous CH4 and NH4+ concentrations at an unsedimented mid-ocean-ridge hydrothermal system. Nature 1993, 364, 45–47. [Google Scholar] [CrossRef]
- Marsh, A.G.; Mullineaux, L.S.; Young, C.M.; Manahan, D.T. Larval dispersal potential of the tubeworm Riftia pachyptila at deep-sea hydrothermal vents. Nature 2001, 411, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Haymon, R.M. Growth history of hydrothermal black smoker chimneys. Nature 1983, 301, 695–698. [Google Scholar] [CrossRef]
- Luff, R.; Wallmann, K. Fluid flow, methane fluxes, carbonate precipitation and biogeochemical turnover in gas hydrate-bearing sediments at Hydrate Ridge, Cascadia Margin: Numerical modeling and mass balances. Geochim. Cosmochim. Acta 2003, 67, 3403–3421. [Google Scholar] [CrossRef]
- Feng, D.; Peng, Y.; Bao, H.; Peckmann, J.; Roberts, H.H.; Chen, D. A carbonate-based proxy for sulfate-driven anaerobic oxidation of methane. Geology 2016, 44, 999–1002. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Chen, D. Authigenic carbonates from an active cold seep of the northern South China Sea: New insights into fluid sources and past seepage activity. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 122, 74–83. [Google Scholar] [CrossRef]
- Burton, E.A. Controls on marine carbonate cement mineralogy: Review and reassessment. Chem. Geol. 1993, 105, 163–179. [Google Scholar] [CrossRef]
- Karan, K.; Yao, X.; Xu, C.; Wang, Y. Chemical profile of the dentin substrate in non-carious cervical lesions. Dent. Mater. 2009, 25, 1205–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Walz, P.M.; Kirkwood, W.J.; Hester, K.C.; Ussler, W.; Peltzer, E.T.; Brewer, P.G. Development and deployment of a deep-sea Raman probe for measurement of pore water geochemistry. Deep Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 297–306. [Google Scholar] [CrossRef]
- Chou, I.M.; Wang, A. Application of laser Raman micro-analyses to Earth and planetary materials. J. Asian Earth Sci. 2017, 145, 309–333. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Z.; Luan, Z.; Wang, X.; Xi, S.; Wang, B.; Li, L.; Lian, C.; Yan, J. In situ Raman Raman Detection of Gas Hydrates Exposed on the Seafloor of the South China Sea. Geochem. Geophys. Geosyst. 2017, 18, 3700–3713. [Google Scholar] [CrossRef]
- Griffith, W.P. Raman Spectroscopy of Minerals. Nature 1969, 224, 264–266. [Google Scholar] [CrossRef]
- Bersani, D.; Andò, S.; Vignola, P.; Moltifiori, G.; Marino, I.G.; Lottici, P.P.; Diella, V. Micro-Raman spectroscopy as a routine tool for garnet analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 484–491. [Google Scholar] [CrossRef]
- Croce, A.; Arrais, A.; Rinaudo, C. Raman Micro-Spectroscopy Identifies Carbonaceous Particles Lying on the Surface of Crocidolite, Amosite, and Chrysotile Fibers. Minerals 2018, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Hou, H.; Liang, H.; Chen, K.; Chen, X. Raman Spectroscopy Study of Phosphorites Combined with PCA-HCA and OPLS-DA Models. Minerals 2019, 9, 578. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Kuebler, K.; Jolliff, B.; Haskin, L.A. Mineralogy of a Martian meteorite as determined by Raman spectroscopy. J. Raman Spectrosc. 2010, 35, 504–514. [Google Scholar] [CrossRef]
- White, S.N. Laser Raman spectroscopy as a technique for identification of seafloor hydrothermal and cold seep minerals. Chem. Geol. 2009, 259, 240–252. [Google Scholar] [CrossRef]
- Xi, S.; Zhang, X.; Du, Z.; Li, L.; Wang, B.; Luan, Z.; Lian, C.; Yan, J. Laser Raman detection of authigenic carbonates from cold seeps at the Formosa Ridge and east of the Pear River Mouth Basin in the South China Sea. J. Asian Earth Sci. 2018, 168, 207–224. [Google Scholar] [CrossRef]
- Freeman, J.J.; Wopenka, B.; Silva, M.J.; Pasteris, J.D. Raman Spectroscopic Detection of Changes in Bioapatite in Mouse Femora as a Function of Age and In Vitro Fluoride Treatment. Calcif. Tissue Int. 2001, 68, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Xueyin, Y.; Chao, G.; Jing, G. An in situ study of the phase transitions among CaCO3 high-pressure polymorphs. Mineral. Mag. 2019, 83, 191–197. [Google Scholar]
- Ono, S.; Kikegawa, T.; Higo, Y. In situ observation of a phase transition in Fe2SiO4 at high pressure and high temperature. Phys. Chem. Miner. 2013, 40, 811–816. [Google Scholar] [CrossRef]
- Bauer, M.; Davydovskaya, P.; Janko, M.; Kaliwoda, M.; Petersen, N.; Gilder, S.; Stark, R.W. Raman spectroscopy of laser-induced oxidation of titanomagnetites. J. Raman Spectrosc. 2011, 42, 1413–1418. [Google Scholar] [CrossRef]
- Weber, I.; Böttger, U.; Pavlov, S.G.; Hübers, H.W.; Hiesinger, H.; Jessberger, E.K. Laser alteration on iron sulfides under various environmental conditions. J. Raman Spectrosc. 2017, 48, 1509–1517. [Google Scholar] [CrossRef]
- Ignatieva, N.; Zakharkina, O.; Leroy, G.; Sobol, E.; Vorobieva, N.; Mordon, S. Molecular processes and structural alterations in laser reshaping of cartilage. Laser Phys. Lett. 2010, 4, 749–753. [Google Scholar] [CrossRef]
- Foucher, F.; Lopez-Reyes, G.; Bost, N.; Rull-Perez, F.; Rüßmann, P.; Westall, F. Effect of grain size distribution on Raman analyses and the consequences for in situ planetary missions. J. Raman Spectrosc. 2013, 44, 916–925. [Google Scholar] [CrossRef] [Green Version]
- Graham, U.M.; Ohmoto, H. Experimental study of formation mechanisms of hydrothermal pyrite. Geochim. Cosmochim. Acta 1994, 58, 2187–2202. [Google Scholar] [CrossRef]
- Kawasumi, S.; Chiba, H.; Kawasumi, S. Redox state of seafloor hydrothermal fluids and its effect on sulfide mineralization. Chem. Geol. 2017, 451, 25–37. [Google Scholar] [CrossRef]
- Janecky, D.R.; Seyfried, W.E., Jr. Formation of massive sulfide deposits on oceanic ridge crests: Incremental reaction models for mixing between hydrothermal solutions and seawater. Geochim. Cosmochim. Acta 1984, 48, 2723–2738. [Google Scholar] [CrossRef]
- Qin, A.M.; Fang, Y.P.; Ou, H.D.; Liu, H.Q.; Su, C.Y. Formation of Various Morphologies of Covellite Copper Sulfide Submicron Crystals by a Hydrothermal Method without Surfactant. Cryst. Growth Des. 2005, 5, 855–860. [Google Scholar] [CrossRef]
- Loyd, S.J.; Sample, J.; Tripati, R.E.; Defliese, W.F.; Brooks, K.; Hovland, M.; Torres, M.; Marlow, J.; Hancock, L.G.; Martin, R. Methane seep carbonates yield clumped isotope signatures out of equilibrium with formation temperatures. Nat. Commun. 2016, 7, 12274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mernagh, T.P.; Trudu, A.G. A laser Raman microprobe study of some geologically important sulphide minerals. Chem. Geol. 1993, 103, 113–127. [Google Scholar] [CrossRef]
- Downs, R.T. Program and Astracts of The 19th General Meeting of the International Mineralogical Association; IMA: Kobe, Japan, 2006; pp. O03–O13. [Google Scholar]
- Mao, B.; Dong, Q.; Exstrom, C.L.; Huang, J. Surface thermal stability of iron pyrite nanocrystals: Role of capping ligands. Thin Solid Film 2014, 562, 361–366. [Google Scholar] [CrossRef]
- Reiss, H. The Fermi level and the redox potential. J. Phys. Chem. 1985, 89, 3783–3791. [Google Scholar] [CrossRef]
- Wood, A.; Giersig, M.; Mulvaney, P. Fermi Level Equilibration in Quantum Dot−Metal Nanojunctions. J. Phys. Chem. B 2001, 105, 8810–8815. [Google Scholar] [CrossRef]
- Ghatak, K.P.; Mondal, M. Theoretical study of the effective electron mass in ternary chalcopyrite semiconductors in the presence of crossed electric and magnetic fields. Z. Phys. B Condens. Matter 1988, 69, 471–479. [Google Scholar] [CrossRef]
- Cao, R.; Miyano, K.; Kendelewicz, T.; Lindau, I.; Spicer, W.E. Low-temperature alkali metal/III–V interfaces: A study of metallization and Fermi level movement. J. Vac. Sci. Technol. B Microelectron. Process. Phenom. 1989, 7, 919–924. [Google Scholar] [CrossRef]
- Dunn, J.G.; Muzenda, C. Thermal oxidation of covellite (CuS). Thermochim. Acta 2001, 369, 117–123. [Google Scholar] [CrossRef]
- Mitchell, R.E. Mechanisms of Pyrite Oxidation to Non-Slagging Species; Final Report; US Department of Energy: Washington, DC, USA, 2002.
- Chen, J.H.; Zhong, J.L.; Li, Y.Q.; Chen, Y.; Guo, J. Electronic structures and floatability of pyrite, marcasite and pyrrhotite. Chin. J. Nonferrous Met. 2011, 21, 1719–1727. [Google Scholar]
- Krishnamurti, D. The Raman spectra of crystalline sulphates of Ni and Mn. Proc. Indian Acad. Sci. Sect. A 1958, 48, 355–363. [Google Scholar] [CrossRef]
- Houk, K.N. Frontier molecular orbital theory of cycloaddition reactions. Acc. Chem. Res. 1975, 8, 361–369. [Google Scholar] [CrossRef]
- Burdon, J.; Parsons, I.W. A recent attempt to apply frontier orbital theory to nucleophilic aromatic substitution. J. Am. Chem. Soc. 1994, 22, 1724–1734. [Google Scholar] [CrossRef]
- Dannenberg, J.J. Using Perturbation and Frontier Molecular Orbital Theory to Predict Diastereofacial Selectivity. Chem. Rev. 1999, 99, 1225–1242. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, J.H.; Chen, Y.; Guo, J. Density functional theory study of influence of impurity on electronic properties and reactivity of pyrite. Trans. Nonferrous Met. Soc. China 2011, 21, 1887–1895. [Google Scholar] [CrossRef]
- Chen, J.H.; Wang, J.M.; Long, X.H.; Guo, J. First-principle theory on electronic structure of copper sulfides. J. Cent. South Univ. 2011, 42, 3612–3617. [Google Scholar]
- Matsumoto, Y.; Harada, H.; Yui, K.; Uchida, H.; Itatani, K.; Koda, S. Raman spectroscopic study of aqueous alkali sulfate solutions at high temperature and pressure to yield precipitation. J. Supercrit. Fluids 2009, 49, 303–309. [Google Scholar] [CrossRef]
- Hapanowicz, R.P.; Condratesr, R.A. High-Temperature Raman Spectral Investigation of Sodium Sulfate. Spectrosc. Lett. 1996, 29, 133–141. [Google Scholar] [CrossRef]
- Bischoff, W.D.; Sharma, S.K.; Mackenzie, F.T. Carbonate ion disorder in synthetic and biogenic magnesian calcites: A Raman spectral study. Plast. Reconstr. Surg. 1985, 112, 489–494. [Google Scholar]
- Mackenzie, F.T.; Urmos, J.; Sharma, S.K. Characterization of some biogenic carbonates with Raman spectroscopy. Am. Mineral. 1991, 76, 641–646. [Google Scholar]
- Gruver, R.M. Differential Thermal-Analysis Studies of Ceramic Materials: II, Transition of Aragonite to Calcite. J. Am. Ceram. Soc. 2010, 33, 171–174. [Google Scholar] [CrossRef]











| Sample ID | Mineral | Type | Location | Formation Temperature |
|---|---|---|---|---|
| YC | Chalcopyrite | VMS | Sampling-4 | High temperature (>300 °C) [29,30,31] |
| DZ3 | Pyrite | VMS | Sampling-3 | Moderate1ow temperature (100–200 °C) [29,30,31] |
| YC | Covellite | VMS | Sampling-3 | Moderate–low temperature (120–180 °C) [32] |
| Ro30 | Barite | Sulfate mineral | Sampling-2 | Low temperature (<100 °C) [31] |
| CS1 | Aragonite | Carbonate mineral | Sampling-1 | Low temperature (<52 °C) [33] |
| Mineral | EHOMO/eV | ELUMO/eV | |ΔE1| |
|---|---|---|---|
| Chalcopyrite | −5.622 | −4.883 | 1.022 |
| Pyrite | −6.477 | −5.732 | 1.837 |
| Covellite | −3.096 | −1.887 | 1.504 |
| Oxygen | −6.900 | −4.610 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, S.; Zhang, X.; Luan, Z.; Du, Z.; Li, L.; Liang, Z.; Lian, C.; Yan, J. Micro-Raman Study of Thermal Transformations of Sulfide and Oxysalt Minerals Based on the Heat Induced by Laser. Minerals 2019, 9, 751. https://doi.org/10.3390/min9120751
Xi S, Zhang X, Luan Z, Du Z, Li L, Liang Z, Lian C, Yan J. Micro-Raman Study of Thermal Transformations of Sulfide and Oxysalt Minerals Based on the Heat Induced by Laser. Minerals. 2019; 9(12):751. https://doi.org/10.3390/min9120751
Chicago/Turabian StyleXi, Shichuan, Xin Zhang, Zhendong Luan, Zengfeng Du, Lianfu Li, Zhengwei Liang, Chao Lian, and Jun Yan. 2019. "Micro-Raman Study of Thermal Transformations of Sulfide and Oxysalt Minerals Based on the Heat Induced by Laser" Minerals 9, no. 12: 751. https://doi.org/10.3390/min9120751