Characterization of Active Electrode Yield for Intracortical Arrays: Awake versus Anesthesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Implantation of Neural Devices
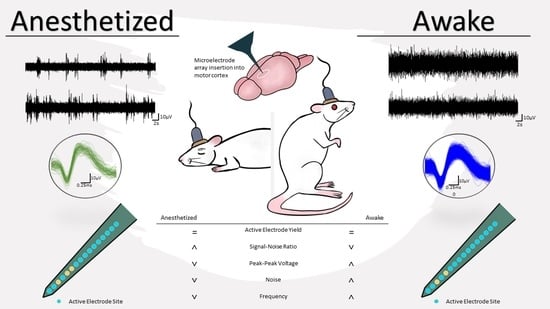
2.2. Neurophysiogical Recording and Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gunasekera, B.; Saxena, T.; Bellamkonda, R.; Karumbaiah, L. Intracortical recording interfaces: Current challenges to chronic recording function. ACS Chem. Neurosci. 2015, 6, 68–83. [Google Scholar] [CrossRef]
- Andrew, J.S.; Jeffrey, R.C. Bioinspired materials and systems for neural interfacing. Curr. Opin. Biomed. Eng. 2018, 6, 110–119. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Jorfi, M.; Skousen, J.L.; Weder, C.; Capadona, J.R. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural. Eng. 2015, 12, 011001. [Google Scholar] [CrossRef]
- Ereifej, E.S.; Rial, G.M.; Hermann, J.K.; Smith, C.S.; Meade, S.M.; Rayyan, J.M.; Chen, K.; Feng, H.; Capadona, J.R. Implantation of Neural Probes in the Brain Elicits Oxidative Stress. Front. Bioeng. Biotechnol. 2018, 6, 9. [Google Scholar] [CrossRef]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural. Eng. 2013, 10, 066014. [Google Scholar] [CrossRef]
- Usoro, J.O.; Sturgill, B.S.; Musselman, K.C.; Capadona, J.R.; Pancrazio, J.J. Intracortical Microelectrode Array Unit Yield under Chronic Conditions: A Comparative Evaluation. Micromachines 2021, 12, 972. [Google Scholar] [CrossRef]
- Deku, F.; Cohen, Y.; Joshi-Imre, A.; Kanneganti, A.; Gardner, T.J.; Cogan, S.F. Amorphous silicon carbide ultramicroelectrode arrays for neural stimulation and recording. J. Neural. Eng. 2018, 15, 016007. [Google Scholar] [CrossRef]
- Geramifard, N.; Dousti, B.; Nguyen, C.K.; Abbott, J.R.; Cogan, S.; Varner, V. Insertion mechanics of amorphous SiC ultra-micro scale neural probes. J. Neural. Eng. 2022. [Google Scholar] [CrossRef]
- Stiller, A.M.; Usoro, J.; Frewin, C.L.; Danda, V.R.; Ecker, M.; Joshi-Imre, A.; Musselman, K.C.; Voit, W.; Modi, R.; Pancrazio, J.J.; et al. Chronic Intracortical Recording and Electrochemical Stability of Thiol-ene/Acrylate Shape Memory Polymer Electrode Arrays. Micromachines 2018, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- Stiller, A.M.; Usoro, J.O.; Lawson, J.; Araya, B.; Gonzalez-Gonzalez, M.A.; Danda, V.R.; Voit, W.E.; Black, B.J.; Pancrazio, J.J. Mechanically Robust, Softening Shape Memory Polymer Probes for Intracortical Recording. Micromachines 2020, 11, 619. [Google Scholar] [CrossRef]
- Sridharan, A.; Muthuswamy, J. Soft, Conductive, Brain-Like, Coatings at Tips of Microelectrodes Improve Electrical Stability under Chronic, In Vivo Conditions. Micromachines 2021, 12, 761. [Google Scholar] [CrossRef]
- Sridharan, A.; Nguyen, J.K.; Capadona, J.R.; Muthuswamy, J. Compliant intracortical implants reduce strains and strain rates in brain tissue in vivo. J. Neural. Eng. 2015, 12, 036002. [Google Scholar] [CrossRef] [Green Version]
- Potter, K.A.; Buck, A.C.; Self, W.K.; Callanan, M.E.; Sunil, S.; Capadona, J.R. The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes. Biomaterials 2013, 34, 7001–7015. [Google Scholar] [CrossRef]
- Golabchi, A.; Wu, B.; Li, X.; Carlisle, D.L.; Kozai, T.D.Y.; Friedlander, R.M.; Cui, X.T. Melatonin improves quality and longevity of chronic neural recording. Biomaterials 2018, 180, 225–239. [Google Scholar] [CrossRef]
- Rennaker, R.L.; Miller, J.; Tang, H.; Wilson, D.A. Minocycline increases quality and longevity of chronic neural recordings. J. Neural. Eng. 2007, 4, L1–L5. [Google Scholar] [CrossRef] [Green Version]
- Potter-Baker, K.A.; Nguyen, J.K.; Kovach, K.M.; Gitomer, M.M.; Srail, T.W.; Stewart, W.G.; Skousen, J.L.; Capadona, J.R. Development of Superoxide Dismutase Mimetic Surfaces to Reduce Accumulation of Reactive Oxygen Species for Neural Interfacing Applications. J. Mater. Chem. B 2014, 2, 2248–2258. [Google Scholar] [CrossRef] [Green Version]
- Usoro, J.O.; Dogra, K.; Abbott, J.R.; Radhakrishna, R.; Cogan, S.F.; Pancrazio, J.J.; Patnaik, S.S. Influence of Implantation Depth on the Performance of Intracortical Probe Recording Sites. Micromachines 2021, 12, 1158. [Google Scholar] [CrossRef]
- Aksenov, D.P.; Miller, M.J.; Dixon, C.J.; Wyrwicz, A.M. The effect of sevoflurane and isoflurane anesthesia on single unit and local field potentials. Exp. Br. Res. 2019, 237, 1521–1529. [Google Scholar] [CrossRef]
- Ou, M.; Zhao, W.; Liu, J.; Liang, P.; Huang, H.; Yu, H.; Zhu, T.; Zhou, C. The General Anesthetic Isoflurane Bilaterally Modulates Neuronal Excitability. iScience 2020, 23, 100760. [Google Scholar] [CrossRef] [Green Version]
- Sorrenti, V.; Cecchetto, C.; Maschietto, M.; Fortinguerra, S.; Buriani, A.; Vassanelli, S. Understanding the Effects of Anesthesia on Cortical Electrophysiological Recordings: A Scoping Review. Int. J. Mol. Sci. 2021, 22, 1286. [Google Scholar] [CrossRef]
- Newberg, L.A.; Milde, J.H.; Michenfelder, J.D. The cerebral metabolic effects of isoflurane at and above concentrations that suppress cortical electrical activity. Anesthesiology 1983, 59, 23–28. [Google Scholar] [CrossRef]
- Detsch, O.; Vahle-Hinz, C.; Kochs, E.; Siemers, M.; Bromm, B. Isoflurane induces dose-dependent changes of thalamic somatosensory information transfer. Br. Res. 1999, 829, 77–89. [Google Scholar] [CrossRef]
- Ries, C.R.; Puil, E. Isoflurane prevents transitions to tonic and burst firing modes in thalamic neurons. Neurosci. Lett. 1993, 159, 91–94. [Google Scholar] [CrossRef]
- Sitdikova, G.; Zakharov, A.; Janackova, S.; Gerasimova, E.; Lebedeva, J.; Inacio, A.R.; Zaynutdinova, D.; Minlebaev, M.; Holmes, G.L.; Khazipov, R. Isoflurane suppresses early cortical activity. Ann. Clin. Transl. Neurol. 2014, 1, 15–26. [Google Scholar] [CrossRef]
- Noda, T.; Takahashi, H. Anesthetic effects of isoflurane on the tonotopic map and neuronal population activity in the rat auditory cortex. Eur. J. Neurosci. 2015, 42, 2298–2311. [Google Scholar] [CrossRef]
- Cecchetto, C.; Vassanelli, S.; Kuhn, B. Simultaneous Two-Photon Voltage or Calcium Imaging and Multi-Channel Local Field Potential Recordings in Barrel Cortex of Awake and Anesthetized Mice. Front. Neurosci. 2021, 15, 741279. [Google Scholar] [CrossRef]
- Mierzejewski, M.; Steins, H.; Kshirsagar, P.; Jones, P.D. The noise and impedance of microelectrodes. J. Neural. Eng. 2020, 17, 052001. [Google Scholar] [CrossRef] [PubMed]
- Michelson, N.J.; Kozai, T.D.Y. Isoflurane and ketamine differentially influence spontaneous and evoked laminar electrophysiology in mouse V1. J. Neurophysiol. 2018, 120, 2232–2245. [Google Scholar] [CrossRef]
- Lindsly, C.; Gonzalez-Islas, C.; Wenner, P. Elevated intracellular Na(+) concentrations in developing spinal neurons. J. Neurochem. 2017, 140, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Michelson, N.J.; Vazquez, A.L.; Eles, J.R.; Salatino, J.W.; Purcell, E.K.; Williams, J.J.; Cui, X.T.; Kozai, T.D.Y. Multi-scale, multi-modal analysis uncovers complex relationship at the brain tissue-implant neural interface: New emphasis on the biological interface. J. Neural. Eng. 2018, 15, 033001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, H.; Marzullo, T.C.; Kipke, D.R. Lower layers in the motor cortex are more effective targets for penetrating microelectrodes in cortical prostheses. J. Neural. Eng. 2009, 6, 026004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Z.; Liu, H.; Xie, F.; Fischer, S.; Adkins, R.S.; Aldridge, A.I.; Ament, S.A.; Bartlett, A.; Behrens, M.M.; Van den Berge, K.; et al. A transcriptomic and epigenomic cell atlas of the mouse primary motor cortex. Nature 2021, 598, 103–110. [Google Scholar] [CrossRef] [PubMed]



| Condition Showing Activity | AEY (%) at Early Time Point (n = 9) | AEY (%) at Chronic Time Point (n = 8) |
|---|---|---|
| Anesthetized and Awake | 63.2 | 31.3 |
| Anesthetized Only | 9.7 | 3.1 |
| Awake Only | 4.9 | 7.0 |
| Neither | 22.2 | 58.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturgill, B.; Radhakrishna, R.; Thai, T.T.D.; Patnaik, S.S.; Capadona, J.R.; Pancrazio, J.J. Characterization of Active Electrode Yield for Intracortical Arrays: Awake versus Anesthesia. Micromachines 2022, 13, 480. https://doi.org/10.3390/mi13030480
Sturgill B, Radhakrishna R, Thai TTD, Patnaik SS, Capadona JR, Pancrazio JJ. Characterization of Active Electrode Yield for Intracortical Arrays: Awake versus Anesthesia. Micromachines. 2022; 13(3):480. https://doi.org/10.3390/mi13030480
Chicago/Turabian StyleSturgill, Brandon, Rahul Radhakrishna, Teresa Thuc Doan Thai, Sourav S. Patnaik, Jeffrey R. Capadona, and Joseph J. Pancrazio. 2022. "Characterization of Active Electrode Yield for Intracortical Arrays: Awake versus Anesthesia" Micromachines 13, no. 3: 480. https://doi.org/10.3390/mi13030480