Antimicrobial Activity of Silver Containing Crosslinked Poly(Acrylic Acid) Fibers
Abstract
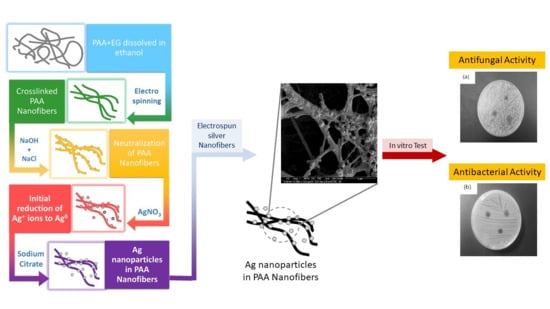
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of PAA Ethanol Solution, PAA Nanofibers, and Thermal Crosslinking of Electrospun PAA Nanofiber (Step I)
2.2.2. Neutralization of PAA Nanofibers (Step II)
2.2.3. Preparation of PAA Nanofibers with Silver Ions (Step III)
2.2.4. Preparation of Hybrid of Silver-PAA Nanofibers (Step IV)
2.3. Characterization
2.4. Antifungal and Antimicrobial Testing
3. Results and Discussion

3.1. Morphology and Structural Characterization
3.2. Optical Characterization
3.3. XRD Study
3.4. XPS Study
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Pazos-Ortiz, E.; Roque-Ruiz, J.H.; Hinojos-Márquez, E.A.; López-Esparza, J.; Donohué-Cornejo, A.; Cuevas González, J.C.; Espinosa Cristóbal, L.F.; Reyes Lopez, S.Y. Dose-dependent antimicrobial activity of silver nanoparticles on polycaprolactone fibers against gram-positive and gram-negative bacteria. J. Nanomater. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Antimicrobial Resistance: Global Report on Surveillance 2014. Available online: http://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed on 27 November 2019).
- Antimicrobial Resistance: Tackling a Crisis for the Future Health and Wealth of Nations. 2014. Available online: http://amr-review.org/ (accessed on 27 November 2019).
- Mofidfar, M.; Wang, J.; Long, L.; Hager, C.L.; Vareechon, C.; Pearlman, E.; Baer, E.; Ghannoum, M.; Wnek, G.E. Polymeric nanofiber/antifungal formulations using a novel co-extrusion approach. AAPS PharmSciTech 2017, 18, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Obregón, R.; Ramón-Azcón, J.; Salazar, G.; Ramalingam, M. Clinical/preclinical aspects of nanofiber composites. In Nanofiber Composites for Biomedical Applications; Ramalingam, M., Ramakrishna, B.A., Eds.; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260. [Google Scholar] [CrossRef]
- Shi, G.; Chen, W.; Zhang, Y.; Dai, X.; Zhang, X.; Wu, Z. An antifouling hydrogel containing silver nanoparticles for modulating the therapeutic immune response in chronic wound healing. Langmuir 2019, 35, 1837–1845. [Google Scholar] [CrossRef]
- Maliszewska, I.; Sadowski, Z. Synthesis and antibacterial activity of silver nanoparticles. J. Phys. Conf. Ser. 2009, 146, 12024. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Fong, J.; Wood, F. Nanocrystalline silver dressings in wound management: A review. Int. J. Nanomed. 2006, 1, 441–449. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, J.H. Preparation and properties of antibacterial poly(vinyl alcohol) nanofibers by nanoparticles. Fiber. Polym. 2011, 12, 602. [Google Scholar] [CrossRef]
- Hong, K.H.; Park, J.L.; Sul, I.H.; Youk, J.H.; Kang, T.J. Preparation of antimicrobial poly(vinyl alcohol) nanofibers containing silver nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2468–2474. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Na, H. Preparation of nanosilver particles into sulfonated poly(ether ether ketone) (S-PEEK) nanostructures by electrospinning. Mater. Lett. 2007, 61, 421–426. [Google Scholar] [CrossRef]
- Jin, W.-J.; Lee, H.K.; Jeong, E.H.; Park, W.H.; Youk, J.H. Preparation of polymer nanofibers containing silver nanoparticles by using poly(n-vinylpyrrolidone). Macromol. Rapid Commun. 2005, 26, 1903–1907. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol. Rapid Commun. 2004, 25, 1632–1637. [Google Scholar] [CrossRef]
- Mansour, H.M.; Rhee, Y.-S.; Wu, X. Nanomedicine in pulmonary delivery. Int. J. Nanomed. 2009, 4, 299–319. [Google Scholar] [CrossRef]
- Santos-Magalhães, N.S.; Mosqueira, V.C.F. Nanotechnology applied to the treatment of malaria. Adv. Drug Deliv. Rev. 2010, 62, 560–575. [Google Scholar] [CrossRef]
- Sosnik, A.; Carcaboso, Á.M.; Glisoni, R.J.; Moretton, M.A.; Chiappetta, D.A. New old challenges in tuberculosis: Potentially effective nanotechnologies in drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 547–559. [Google Scholar] [CrossRef]
- Sureshkumar, M.; Siswanto, D.Y.; Lee, C.-K. Magnetic antimicrobial nanocomposite based on bacterial cellulose and silver nanoparticles. J. Mater. Chem. 2010, 20, 6948–6955. [Google Scholar] [CrossRef]
- Pacioni, N.L.; Borsarelli, C.D.; Rey, V.; Veglia, A.V. Synthetic Routes for the Preparation of Silver Nanoparticles. In Silver Nanoparticle Applications: In Fabrication and Design of Medical and Biosensing Devices; Alarcon, E.I., Griffith, M., Udekwu, K.I., Eds.; Springer: Berlin, Germany, 2015. [Google Scholar]
- Pillai, Z.S.; Kamat, P.V. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J. Phys. Chem. B 2004, 108, 945–951. [Google Scholar] [CrossRef]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Zamarreño, C.R.; Arregui, F.J.; Matías, I.R. An antibacterial coating based on a polymer/sol-gel hybrid matrix loaded with silver nanoparticles. Nanoscale Res. Lett. 2011, 6, 305. [Google Scholar] [CrossRef]
- Henglein, A. Physicochemical properties of small metal particles in solution: “Microelectrode” reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J. Phys. Chem. 1993, 97, 5457–5471. [Google Scholar] [CrossRef]
- González-Sánchez, M.I.; Perni, S.; Tommasi, G.; Morris, N.G.; Hawkins, K.; López-Cabarcos, E.; Prokopovich, P. Silver nanoparticle based antibacterial methacrylate hydrogels potential for bone graft applications. Mater. Sci. Eng. C 2015, 50, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Fu, J.; Lin, C.; Lin, J.; Han, Y.; Yu, X.; Pan, C. Formation and photoluminescence of silver nanoparticles stabilized by a two-armed polymer with a crown ether core. Langmuir 2004, 20, 9775–9779. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, C. Seed-mediated growth technique for the preparation of a silver nanoshell on a silica sphere. J. Phys. Chem. B 2003, 107, 12411–12415. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Droubay, T.; Chambers, S.A.; Bagus, P.S. Spectroscopic evidence for Ag(III) in highly oxidized silver films by X-ray photoelectron spectroscopy. J. Phys. Chem. C 2010, 114, 21562–21571. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. JBIC J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Vu, N.K.; Zille, A.; Oliveira, F.R.; Carneiro, N.; Souto, A.P. Effect of particle size on silver nanoparticle deposition onto dielectric barrier discharge (DBD) plasma functionalized polyamide fabric. Plasma Process. Polym. 2013, 10, 285–296. [Google Scholar] [CrossRef]
- Briggs, D. Handbook of X-ray photoelectron spectroscopy C.D.; Wanger, W.M.; Riggs, L.E.; Davis, J.F. Moulder and G.E. Muilenberg Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA, 1979. 190 pp. $ 195. Surf. Interface Anal. 1981, 3. [Google Scholar] [CrossRef]
- Schlosser, M.; Fröls, S.; Hauf, U.; Sethmann, I.; Schultheiss, S.; Pfeifer, F.; Kleebe, H. Combined hydrothermal conversion and vapor transport sintering of Ag-modified calcium phosphate scaffolds. J. Am. Ceram. Soc. 2013, 96, 412–419. [Google Scholar] [CrossRef]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria, nanomedicine nanotechnology. Biol. Med. 2012, 8, 37–45. [Google Scholar]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]







| C 1s | O 1s | Ag 3d | Na 1s | Cl 2p | S 2p |
|---|---|---|---|---|---|
| 63.2 | 27.4 | 5.4 | 3.5 | 0.3 | 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mofidfar, M.; Kim, E.S.; Larkin, E.L.; Long, L.; Jennings, W.D.; Ahadian, S.; Ghannoum, M.A.; Wnek, G.E. Antimicrobial Activity of Silver Containing Crosslinked Poly(Acrylic Acid) Fibers. Micromachines 2019, 10, 829. https://doi.org/10.3390/mi10120829
Mofidfar M, Kim ES, Larkin EL, Long L, Jennings WD, Ahadian S, Ghannoum MA, Wnek GE. Antimicrobial Activity of Silver Containing Crosslinked Poly(Acrylic Acid) Fibers. Micromachines. 2019; 10(12):829. https://doi.org/10.3390/mi10120829
Chicago/Turabian StyleMofidfar, Mohammad, Eun Seon Kim, Emily L. Larkin, Lisa Long, Wayne D. Jennings, Samad Ahadian, Mahmoud A. Ghannoum, and Gary E. Wnek. 2019. "Antimicrobial Activity of Silver Containing Crosslinked Poly(Acrylic Acid) Fibers" Micromachines 10, no. 12: 829. https://doi.org/10.3390/mi10120829