Design of Eutectic Hydrated Salt Composite Phase Change Material with Cement for Thermal Energy Regulation of Buildings
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials
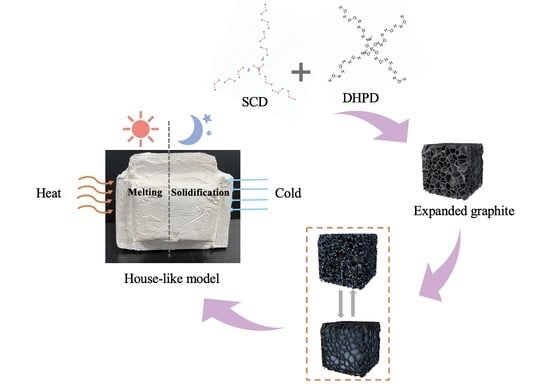
2.2. Preparation of SD/E and MOC
2.3. Characterization
3. Results and Discussion
3.1. Chemical Compatibility Analysis of SD and SD/E
3.2. Microscopic Morphology of the EG and SD/E
3.3. Phase Change Behavior of SD and SD/E
3.3.1. Thermal Storage Parameters of SD and SD/E
3.3.2. Supercooling Behavior of Hydrated Salts and SD/E
3.4. Thermal Conductivities of SD and SD/E
3.5. Experimental Exploration of SD/E for Thermal Energy Storage
4. Conclusions
- Based on the DSC, XRD, and FT-IR results, the latent heat of SD/E was 196.2 J/g while the phase change point was 23.5 °C. The SD/E showed excellent chemical stability, which suggested that SD/E was a promising candidate for thermal energy storage.
- The combination of EG showed an obvious effect on decreasing the supercooling of SD. After the packaging with EG, the supercooling degree dropped from 5.7 °C to 0.8 °C.
- The thermal conductivity of SD was 0.82 W/(m·K), while it improved to 2.74 W/(m·K) after the combination with EG, which indicating the encapsulation of EG could enhance the heat conduction performance obviously.
- Due to the environmental and economic advantages of the experimental explorations, SD/E had a strong insolation prospect for construction materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayyar, M.; Weerasiri, R.R.; Soroushian, P.; Lu, J. Experimental and numerical study of shape-stable phase-change nanocomposite toward energy-efficient building constructions. Energy Build. 2014, 75, 249–255. [Google Scholar] [CrossRef]
- da Cunha, S.R.L.; de Aguiar, J.L.B. Phase change materials and energy efficiency of buildings: A review of knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Xu, G.; Wang, W. China’s energy consumption in construction and building sectors: An outlook to 2100. Energy 2020, 195. [Google Scholar] [CrossRef]
- Marani, A.; Nehdi, M.L. Integrating phase change materials in construction materials: Critical review. Constr. Build. Mater. 2019, 217, 36–49. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Luan, X.; Song, S. Effects of acid leaching and organic intercalation on the thermophysical properties of paraffin/expanded vermiculite composite phase change materials. Appl. Clay Sci. 2020, 196. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Zhang, Y. Review of thermal energy storage technologies based on PCM application in buildings. Energy Build. 2013, 67, 56–69. [Google Scholar] [CrossRef]
- de Gracia, A.; Oró, E.; Farid, M.M.; Cabeza, L.F. Thermal analysis of including phase change material in a domestic hot water cylinder. Appl. Therm. Eng. 2011, 31, 3938–3945. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, W.A.; Nair, N.-K.C.; Farid, M.M. Impact of energy storage in buildings on electricity demand side management. Energy Convers. Manag. 2011, 52, 2110–2120. [Google Scholar] [CrossRef]
- Reddy, K.S.; Mudgal, V.; Mallick, T.K. Review of latent heat thermal energy storage for improved material stability and effective load management. J. Energy Storage 2018, 15, 205–227. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Bolivar Osorio, F.J.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, M.; Fan, J.; Li, L.; Xu, T.; Yuan, W. Thermal conductivity enhancement of hydrated salt phase change materials employing copper foam as the supporting material. Sol. Energy Mater. Sol. Cells 2019, 199, 91–98. [Google Scholar] [CrossRef]
- Fu, W.; Zou, T.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Thermal properties and thermal conductivity enhancement of composite phase change material using sodium acetate trihydrate–urea/expanded graphite for radiant floor heating system. Appl. Therm. Eng. 2018, 138, 618–626. [Google Scholar] [CrossRef]
- Cheng, F.; Zhang, X.; Wen, R.; Huang, Z.; Fang, M.; Liu, Y.g.; Wu, X.; Min, X. Thermal conductivity enhancement of form-stable tetradecanol/expanded perlite composite phase change materials by adding Cu powder and carbon fiber for thermal energy storage. Appl. Therm. Eng. 2019, 156, 653–659. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.A.; Xu, Y.; Dong, J. A review on supercooling of Phase Change Materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Sandnes, B.; Rekstad, J. Supercooling salt hydrates: Stored enthalpy as a function of temperature. Sol. Energy 2006, 80, 616–625. [Google Scholar] [CrossRef]
- Mao, J.; Dong, X.; Hou, P.; Lian, H. Preparation research of novel composite phase change materials based on sodium acetate trihydrate. Appl. Therm. Eng. 2017, 118, 817–825. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Li, X.; Zhou, Y.; Sun, Q.; Yun, Q. The preparation, characterization and modification of a new phase change material: CaCl2·6H2O–MgCl2·6H2O eutectic hydrate salt. Sol. Energy Mater. Sol. Cells 2014, 126, 51–55. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Use of nano-α-Al2O3 to improve binary eutectic hydrated salt as phase change material. Sol. Energy Mater. Sol. Cells 2017, 160, 18–25. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010, 42, 1361–1368. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Li, J.; Qian, T.; Guan, W.; Wang, X. Preparation and Characterization of KNO3/Diatomite Shape-Stabilized Composite Phase Change Material for High Temperature Thermal Energy Storage. J. Mater. Sci. Technol. 2017, 33, 198–203. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Deng, Y.; Guan, W.; Ning, L. Diatomite: A promising natural candidate as carrier material for low, middle and high temperature phase change material. Energy Convers. Manag. 2015, 98, 34–45. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Q.; Ye, R.; Fang, X.; Zhang, Z. A calcium chloride hexahydrate/expanded perlite composite with good heat storage and insulation properties for building energy conservation. Renew. Energy 2017, 114, 733–743. [Google Scholar] [CrossRef]
- Rao, Z.; Xu, T.; Liu, C.; Zheng, Z.; Liang, L.; Hong, K. Experimental study on thermal properties and thermal performance of eutectic hydrated salts/expanded perlite form-stable phase change materials for passive solar energy utilization. Sol. Energy Mater. Sol. Cells 2018, 188, 6–17. [Google Scholar] [CrossRef]
- Xie, N.; Luo, J.; Li, Z.; Huang, Z.; Gao, X.; Fang, Y.; Zhang, Z. Salt hydrate/expanded vermiculite composite as a form-stable phase change material for building energy storage. Sol. Energy Mater. Sol. Cells 2019, 189, 33–42. [Google Scholar] [CrossRef]
- Han, W.; Ge, C.; Zhang, R.; Ma, Z.; Wang, L.; Zhang, X. Boron nitride foam as a polymer alternative in packaging phase change materials: Synthesis, thermal properties and shape stability. Appl. Energy 2019, 238, 942–951. [Google Scholar] [CrossRef]
- Li, T.X.; Wu, D.L.; He, F.; Wang, R.Z. Experimental investigation on copper foam/hydrated salt composite phase change material for thermal energy storage. Int. J. Heat Mass Transf. 2017, 115, 148–157. [Google Scholar] [CrossRef]
- Peng, S.; Huang, J.; Wang, T.; Zhu, P. Effect of fumed silica additive on supercooling, thermal reliability and thermal stability of Na2HPO4·12H2O as inorganic PCM. Thermochim. Acta 2019, 675, 1–8. [Google Scholar] [CrossRef]
- Song, Z.; Deng, Y.; Li, J.; Nian, H. Expanded graphite for thermal conductivity and reliability enhancement and supercooling decrease of MgCl2⋅6H2O phase change material. Mater. Res. Bull. 2018, 102, 203–208. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J.; Chen, Z.; Long, Y. Stearic acid/expanded graphite as a composite phase change thermal energy storage material for tankless solar water heater. Sustain. Cities Soc. 2019, 44, 458–464. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, W.; Zhang, W.; Li, J.; Chen, H. Tuning the phase structure and mechanical performance of magnesium oxychloride cements by curing temperature and H2O/MgCl2 ratio. Constr. Build. Mater. 2018, 179, 413–419. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Tan, J. Heat storage properties of the cement mortar incorporated with composite phase change material. Appl. Energy 2013, 103, 393–399. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X. Experimental investigation on heat transfer characteristics of a new radiant floor system with phase change material. J. Southeast Univ. 2015, 31, 496–500. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation. Sol. Energy Mater. Sol. Cells 2009, 93, 1366–1376. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Nian, H. Polyethylene glycol-enwrapped silicon carbide nanowires network/expanded vermiculite composite phase change materials: Form-stabilization, thermal energy storage behavior and thermal conductivity enhancement. Sol. Energy Mater. Sol. Cells 2018, 174, 283–291. [Google Scholar] [CrossRef]
- Qian, Y.; Meng, B.; Zhang, X.; Zheng, Y.; Taylor, R.; Tiffany-Castiglioni, E. HSPA5 forms specific complexes with copper. Neurochem. Res. 2013, 38, 321–329. [Google Scholar] [CrossRef]
- Burba, C.M.; Janzen, J. Confinement effects on the phase transition temperature of aqueous NaCl solutions: The extended Gibbs–Thomson equation. Thermochim. Acta 2015, 615, 81–87. [Google Scholar] [CrossRef]








| Samples | Melting | Solidification | R(%) | E(%) | φ(%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| TM (°C) | HE (J/g) | HC (J/g) | TS (°C) | HE (J/g) | HC (J/g) | ||||
| SD | 24.2 | 239.4 | 239.4 | 10.5 | 207.4 | 207.4 | — | — | — |
| SD/E | 23.5 | 196.2 | 203.5 | 11.5 | 168.7 | 176.3 | 82.0 | 81.7 | 99.6 |
| Samples | Supercooling Degree (°C) | Suppression Ratio (%) |
|---|---|---|
| DHPD | 11.1 | - |
| SCD | 7.7 | - |
| SD | 5.7 | - |
| SD/E | 0.8 | 86.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Liu, L.; Yang, Z.; Wu, Y.; Li, J. Design of Eutectic Hydrated Salt Composite Phase Change Material with Cement for Thermal Energy Regulation of Buildings. Materials 2021, 14, 139. https://doi.org/10.3390/ma14010139
Wu N, Liu L, Yang Z, Wu Y, Li J. Design of Eutectic Hydrated Salt Composite Phase Change Material with Cement for Thermal Energy Regulation of Buildings. Materials. 2021; 14(1):139. https://doi.org/10.3390/ma14010139
Chicago/Turabian StyleWu, Niuniu, Lijie Liu, Zhiwei Yang, Yifan Wu, and Jinhong Li. 2021. "Design of Eutectic Hydrated Salt Composite Phase Change Material with Cement for Thermal Energy Regulation of Buildings" Materials 14, no. 1: 139. https://doi.org/10.3390/ma14010139