Injectable Xenogeneic Dental Pulp Decellularized Extracellular Matrix Hydrogel Promotes Functional Dental Pulp Regeneration
Abstract
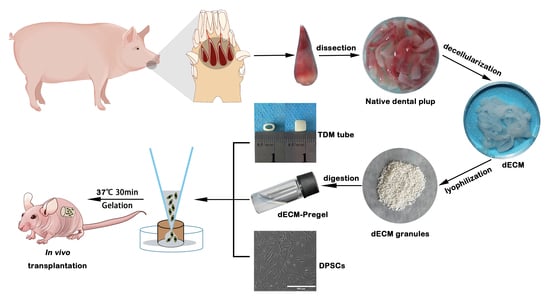
:1. Introduction
2. Results
2.1. Characterization of Porcine Dental Pulp dECM
2.2. Characterization of the dECM Hydrogels
2.3. dECM Hydrogels Demonstrate Favorable Biocompatibility and Chemotactic Activity, Facilitating Cell Survival, Proliferation, and Migration
2.4. dECM Hydrogels Exhibit Strong Angiogenic Properties and Facilitate DPSCs Differentiation into Odontogenesis and Neurogenesis
2.5. dECM Hydrogels Promote Regeneration of Dental Pulp-like Tissue In Vivo
3. Discussion
4. Materials and Methods
4.1. Decellularization of Porcine Dental Pulp
4.2. Characterization of Porcine Dental Pulp dECM
4.3. Fabrication of dECM Hydrogels
4.4. SEM Analysis
4.5. Rheological Characterization
4.6. Swelling and Degradation Analysis In Vitro
4.7. Cell Isolation and Culture
4.8. Cell Proliferation
4.9. Cell Viability
4.10. Cell Migration
4.11. Tube Formation
4.12. Odontogenic and Neurogenic Differentiation
4.13. Preparation of Treated Dentin Matrix (TDM)
4.14. Animal Studies
4.15. Histological Analysis
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmalz, G.; Widbiller, M.; Galler, K.M. Clinical Perspectives of Pulp Regeneration. J. Endod. 2020, 46, S161–S174. [Google Scholar] [CrossRef]
- Yong, D.; Cathro, P. Conservative pulp therapy in the management of reversible and irreversible pulpitis. Aust. Dent. J. 2021, 66, S4–S14. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; El-Karim, I.; Dummer, P.M.H.; Whitworth, J.; Nagendrababu, V. Factors that influence the outcome of pulpotomy in permanent teeth. Int. Endod. J. 2022, 56, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.X.; Yap, A.U. Vital pulp therapy in carious pulp–exposed permanent teeth: An umbrella review. Clin. Oral Investig. 2021, 25, 6743–6756. [Google Scholar] [CrossRef]
- Pereira, A.C.; Oliveira, M.L.; Cerqueira-Neto, A.C.C.L.; Vargas-Neto, J.; Nagata, J.Y.; Gomes, B.P.F.A.; Ferraz, C.C.R.; de Almeida, J.F.A.; de-Jesus-Soares, A. Outcomes of traumatised immature teeth treated with apexification or regenerative endodontic procedure: A retrospective study. Aust. Endod. J. 2020, 47, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Kim, S.; Sun, T.; Cho, Y.-B.; Song, M. Pulp-dentin regeneration: Current approaches and challenges. J. Tissue Eng. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Liao, W.-C.; Chen, C.-H.; Pan, Y.-H.; Chang, M.-C.; Jeng, J.-H. Vertical Root Fracture in Non-Endodontically and Endodontically Treated Teeth: Current Understanding and Future Challenge. J. Pers. Med. 2021, 11, 1375. [Google Scholar] [CrossRef]
- Barnes, J.J.; Patel, S. Contemporary endodontics—Part 1. Br. Dent. J. 2011, 211, 463–468. [Google Scholar] [CrossRef]
- Abbott, P.V. Indications for root canal treatment following traumatic dental injuries to permanent teeth. Aust. Dent. J. 2023, 68, S123–S140. [Google Scholar] [CrossRef]
- Piglionico, S.S.; Pons, C.; Romieu, O.; Cuisinier, F.; Levallois, B.; Panayotov, I.V. In vitro, ex vivo, and in vivo models for dental pulp regeneration. J. Mater. Sci. Mater. Med. 2023, 34, 15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Liu, Y.; Jiang, L.; Wang, X.; Chen, Y.; Li, L.; Song, M.; Zhang, H.; Zhang, Y.S.; Zhang, X. Injectable decellularized dental pulp matrix-functionalized hydrogel microspheres for endodontic regeneration. Acta Biomater. 2023, 156, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Gong, J.; Lu, K.; Hong, Y.; Zhu, Z.; Zhang, J.; Zou, Y.; Zhou, F.; Zhang, C.; Zhou, S.; et al. DLP printed hDPSC-loaded GelMA microsphere regenerates dental pulp and repairs spinal cord. Biomaterials 2023, 299, 122137. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Li, W.; Jiang, W.; Wen, J.; Gu, S. Creating a Microenvironment to Give Wings to Dental Pulp Regeneration—Bioactive Scaffolds. Pharmaceutics 2023, 15, 158. [Google Scholar] [CrossRef]
- Sugiaman, V.K.; Jeffrey; Naliani, S.; Pranata, N.; Djuanda, R.; Saputri, R.I. Polymeric Scaffolds Used in Dental Pulp Regeneration by Tissue Engineering Approach. Polymers 2023, 15, 1082. [Google Scholar] [CrossRef]
- Karobari, M.I.; Parveen, A.; Mirza, M.B.; Makandar, S.D.; Nik Abdul Ghani, N.R.; Noorani, T.Y.; Marya, A. Root and Root Canal Morphology Classification Systems. Int. J. Dent. 2021, 2021, 6682189. [Google Scholar] [CrossRef]
- Gomez, F.; Brea, G.; Gomez-Sosa, J.F. Root canal morphology and variations in mandibular second molars: An in vivo cone-beam computed tomography analysis. BMC Oral Health 2021, 21, 424. [Google Scholar] [CrossRef]
- Samiei, M.; Fathi, M.; Barar, J.; Fathi, N.; Amiryaghoubi, N.; Omidi, Y. Bioactive hydrogel-based scaffolds for the regeneration of dental pulp tissue. J. Drug Deliv. Sci. Technol. 2021, 64, 102600. [Google Scholar] [CrossRef]
- Khayat, A.; Monteiro, N.; Smith, E.E.; Pagni, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. GelMA-Encapsulated hDPSCs and HUVECs for Dental Pulp Regeneration. J. Dent. Res. 2016, 96, 192–199. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, T.; Zhang, R.; Liang, X.; Wang, G.; Tian, Y.; Xie, L.; Tian, W. Platelet lysate functionalized gelatin methacrylate microspheres for improving angiogenesis in endodontic regeneration. Acta Biomater. 2021, 136, 441–455. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Valente, S.; Parchi, G.; Pasquinelli, G.; Taddei, P.; Prati, C. Green Hydrogels Composed of Sodium Mannuronate/Guluronate, Gelatin and Biointeractive Calcium Silicates/Dicalcium Phosphate Dihydrate Designed for Oral Bone Defects Regeneration. Nanomaterials 2021, 11, 3439. [Google Scholar] [CrossRef] [PubMed]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Moshy, S.E.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2020, 42, 107421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Zheng, Z.; Wei, X.; Chen, L.; Wu, Y.; Huang, W.; Yang, L. Strategies for improving the 3D printability of decellularized extracellular matrix bioink. Theranostics 2023, 13, 2562–2587. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liao, L.; Tian, W. Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration. Biomolecules 2023, 13, 673. [Google Scholar] [CrossRef]
- Liu, H.; Gong, Y.; Zhang, K.; Ke, S.; Wang, Y.; Wang, J.; Wang, H. Recent Advances in Decellularized Matrix-Derived Materials for Bioink and 3D Bioprinting. Gels 2023, 9, 195. [Google Scholar] [CrossRef]
- Ungerleider, J.L.; Johnson, T.D.; Rao, N.; Christman, K.L. Fabrication and characterization of injectable hydrogels derived from decellularized skeletal and cardiac muscle. Methods 2015, 84, 53–59. [Google Scholar] [CrossRef]
- Bejleri, D.; Davis, M.E. Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration. Adv. Healthc. Mater. 2019, 8, e1801217. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.; Cha, M.; Kim, S.H.; Jung, Y. The Regeneration of Large-Sized and Vascularized Adipose Tissue Using a Tailored Elastic Scaffold and dECM Hydrogels. Int. J. Mol. Sci. 2021, 22, 12560. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Pezeshki-Modaress, M.; Kiaipour, Z.; Shafiee, M.; Ellini, M.R.; Mazidi, A.; Rajabi, S.; Zamanlui Benisi, S.; Ostad, S.N.; Galler, K.; et al. Pulp ECM-derived macroporous scaffolds for stimulation of dental-pulp regeneration process. Dent. Mater. 2020, 36, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rao, Z.; Zhao, Y.; Xu, Y.; Chen, L.; Shen, Z.; Bai, Y.; Lin, Z.; Huang, Q. A Decellularized Matrix Hydrogel Derived from Human Dental Pulp Promotes Dental Pulp Stem Cell Proliferation, Migration, and Induced Multidirectional Differentiation In vitro. J. Endod. 2020, 46, 1438–1447.e5. [Google Scholar] [CrossRef] [PubMed]
- Joan, K.; Lunney, A.V.G.; Kristen, E. Walker, Taylor Hailstock, Jasmine Franklin, Chaohui Dai, Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar]
- Almarzouqi, F.; Rennekampff, H.O.; Almarzouki, M.; Lambertz, A.; Acharya, M.; Klink, C.; Popov, A.F.; Pallua, N. Porcine-Derived Biomaterials in Tissue Engineering and Reconstructive Surgery: Considerations and Alternatives in Muslim Patients. J. Tissue Eng. Regen. Med. 2018, 13, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Correia, F.; Gouveia, S.A.; Pozza, D.H.; Felino, A.C.; Faria-Almeida, R. A Randomized Clinical Trial Comparing Implants Placed in Two Different Biomaterials Used for Maxillary Sinus Augmentation. Materials 2023, 16, 1220. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Scarano, A.; Piattelli, M.; Piccirilli, M.; Caputi, S.; Piattelli, A. Histologic and Ultrastructural Analysis of Regenerated Bone in Maxillary Sinus Augmentation Using a Porcine Bone–Derived Biomaterial. J. Periodontol. 2006, 77, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, R.; Testarelli, L.; Stefanelli, L.; De Angelis, F.; Mencio, F.; Pompa, G.; Ddi Carlo, S. Bone Healing in Extraction Sockets Covered With Collagen Membrane Alone or Associated With Porcine-Derived Bone Graft: A Comparative Histological and Histomorphometric Analysis. J. Oral Maxillofac. Res. 2017, 8, e1. [Google Scholar] [CrossRef] [PubMed]
- Sapoznikov, L.; Haim, D.; Zavan, B.; Scortecci, G.; Humphrey, M.F. A novel porcine dentin-derived bone graft material provides effective site stability for implant placement after tooth extraction: A randomized controlled clinical trial. Clin. Oral Investig. 2023, 27, 2899–2911. [Google Scholar] [CrossRef]
- Wong, M.L.; Griffiths, L.G. Immunogenicity in xenogeneic scaffold generation: Antigen removal vs. decellularization. Acta Biomater. 2014, 10, 1806–1816. [Google Scholar] [CrossRef]
- Morticelli, L.; Magdei, M.; Tschalaki, N.; Petersen, B.; Haverich, A.; Hilfiker, A. Generation of glycans depleted decellularized porcine pericardium, using digestive enzymatic supplements and enzymatic mixtures for food industry. Xenotransplantation 2021, 28, e12705. [Google Scholar] [CrossRef]
- Hodde, J.; Hiles, M. Virus safety of a porcine-derived medical device: Evaluation of a viral inactivation method. Biotechnol. Bioeng. 2002, 79, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Sharifi, R.; Mamodaly, S.; Islam, R.; Nahra, D.; Abusamra, D.B.; Hui, P.C.; Adibnia, Y.; Goulamaly, M.; Paschalis, E.I.; et al. Effects of gamma radiation sterilization on the structural and biological properties of decellularized corneal xenografts. Acta Biomater. 2019, 96, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.X.; Huang, Y.; Li, K.; Fan, Y.B.; Xie, H.Q.; Li, X.M. Small intestinal submucosa: Superiority, limitations and solutions, and its potential to address bottlenecks in tissue repair. J. Mater. Chem. B 2019, 7, 5038–5055. [Google Scholar] [CrossRef]
- Sammartino, G.; Tia, M.; Gentile, E.; Marenzi, G.; Claudio, P.P. Platelet-Rich Plasma and Resorbable Membrane for Prevention of Periodontal Defects After Deeply Impacted Lower Third Molar Extraction. J. Oral Maxillofac. Surg. 2009, 67, 2369–2373. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Mao, J.; Yang, G.; Wang, H. Influence of different incision designs on bone increment of guided bone regeneration (Bio-Gide collagen membrane +Bio-OSS bone powder) during the same period of maxillary anterior tooth implantation. Bioengineered 2021, 12, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Liu, K.; Liu, R.-X.; Xu, B.-H. Safety and Efficacy of Midface Augmentation Using Bio-Oss Bone Powder and Bio-Gide Collagen Membrane in Asians. J. Clin. Med. 2023, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Jiang, C.; Guo, H.; Luo, G.; Huang, Y.; Yuan, C. Clinical applications of concentrated growth factors membrane for sealing the socket in alveolar ridge preservation: A randomized controlled trial. Int. J. Implant Dent. 2022, 8, 46. [Google Scholar] [CrossRef]
- Hu, L.; Gao, Z.; Xu, J.; Zhu, Z.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Decellularized Swine Dental Pulp as a Bioscaffold for Pulp Regeneration. BioMed Res. Int. 2017, 2017, 9342714. [Google Scholar] [CrossRef]
- Alqahtani, Q.; Zaky, S.H.; Patil, A.; Beniash, E.; Ray, H.; Sfeir, C. Decellularized Swine Dental Pulp Tissue for Regenerative Root Canal Therapy. J. Dent. Res. 2018, 97, 1460–1467. [Google Scholar] [CrossRef]
- Fu, J.; Chen, J.; Li, W.; Yang, X.; Yang, J.; Quan, H.; Huang, H.; Chen, G. Laminin-Modified Dental Pulp Extracellular Matrix for Dental Pulp Regeneration. Front. Bioeng. Biotechnol. 2021, 8, 595096. [Google Scholar] [CrossRef]
- Traphagen, S.B.; Fourligas, N.; Xylas, J.F.; Sengupta, S.; Kaplan, D.L.; Georgakoudi, I.; Yelick, P.C. Characterization of natural, decellularized and reseeded porcine tooth bud matrices. Biomaterials 2012, 33, 5287–5296. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, M.; Zhang, Q.; Zhang, T.; Tian, T.; Ma, Q.; Xue, C.; Lin, S.; Cai, X. Stiffness regulates the proliferation and osteogenic/odontogenic differentiation of human dental pulp stem cells via the WNT signalling pathway. Cell Prolif. 2018, 51, e12435. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hou, Y.; Chu, Z.; Wei, Q. Soft overcomes the hard: Flexible materials adapt to cell adhesion to promote cell mechanotransduction. Bioact. Mater. 2022, 10, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.; Tahmasebi Birgani, Z.; Habibovic, P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials 2022, 283, 121431. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yuan, X.; Zhao, Y.; Cai, Q.; Wang, Y.; Luo, R.; Yu, S.; Wang, Y.; Han, J.; Ge, L.; et al. Injectable GelMA Cryogel Microspheres for Modularized Cell Delivery and Potential Vascularized Bone Regeneration. Small 2021, 17, 2006596. [Google Scholar] [CrossRef]
- Cao, M.; Zhou, Y.; Mao, J.; Wei, P.; Chen, D.; Wang, R.; Cai, Q.; Yang, X. Promoting osteogenic differentiation of BMSCs via mineralization of polylactide/gelatin composite fibers in cell culture medium. Mater. Sci. Eng. C 2019, 100, 862–873. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2018, 13, 58–75. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Zhang, W.; Du, A.; Liu, S.; Lv, M.; Chen, S. Research progress in decellularized extracellular matrix-derived hydrogels. Regen. Ther. 2021, 18, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Roy, S.; Ghosh, S. Regulation of decellularized matrix mediated immune response. Biomater. Sci. 2020, 8, 1194–1215. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef]
- White, L.J.; Taylor, A.J.; Faulk, D.M.; Keane, T.J.; Saldin, L.T.; Reing, J.E.; Swinehart, I.T.; Turner, N.J.; Ratner, B.D.; Badylak, S.F. The impact of detergents on the tissue decellularization process: A ToF-SIMS study. Acta Biomater. 2017, 50, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, E.E.; Lanier, S.T.; Niknam-Bienia, S.; Arenas, G.A.; Rajendran, D.; Wertheim, J.A.; Galiano, R.D. Residual sodium dodecyl sulfate in decellularized muscle matrices leads to fibroblast activation in vitro and foreign body response in vivo. J. Tissue Eng. Regen. Med. 2017, 12, E1704–E1715. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Rajabi, S.; Pezeshki-Modaress, M.; Ellini, M.R.; Panahinia, M.; Alijani, S.; Mazidi, A.; Kamali, A.; Azarpazhooh, A.; Kishen, A. Optimizing Methods for Bovine Dental Pulp Decellularization. J. Endod. 2021, 47, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, W.A. Analysis of collagen, glycoproteins and acid mucopolysaccharides in the bovine and porcine dental pulp. Arch. Oral Biol. 1974, 19, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable scaffolds: Preparation and application in dental and craniofacial regeneration. Mater. Sci. Eng. R Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef]
- Qiu, P.; Li, M.; Chen, K.; Fang, B.; Chen, P.; Tang, Z.; Lin, X.; Fan, S. Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis. Biomaterials 2020, 227, 119552. [Google Scholar] [CrossRef]
- Tsui, J.H.; Leonard, A.; Camp, N.D.; Long, J.T.; Nawas, Z.Y.; Chavanachat, R.; Smith, A.S.T.; Choi, J.S.; Dong, Z.; Ahn, E.H.; et al. Tunable electroconductive decellularized extracellular matrix hydrogels for engineering human cardiac microphysiological systems. Biomaterials 2021, 272, 120764. [Google Scholar] [CrossRef]
- Yan, M.; Li, B.; Zhao, X.; Qin, S. Effect of concentration, pH and ionic strength on the kinetic self-assembly of acid-soluble collagen from walleye pollock (Theragra chalcogramma) skin. Food Hydrocoll. 2012, 29, 199–204. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Wang, Y.; Sun, X.; Li, B.; Poungchawanwong, S.; Hou, H. Structural feature and self-assembly properties of type II collagens from the cartilages of skate and sturgeon. Food Chem. 2020, 331, 127340. [Google Scholar] [CrossRef]
- Tayabally, S.E.H.; Khan, A.A.; Abdallah, S.H.; Khattak, M.N.K.; Jayakumar, M.N.; Rani Samsudin, A.B. Increased strength in the Col-Tgel induces apoptosis in the human dental pulp stem cells: 3D culturing of human dental pulp stem cells at different strengths of collagen. Saudi J. Biol. Sci. 2022, 29, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Goldshmid, R.; Seliktar, D. Hydrogel Modulus Affects Proliferation Rate and Pluripotency of Human Mesenchymal Stem Cells Grown in Three-Dimensional Culture. ACS Biomater. Sci. Eng. 2017, 3, 3433–3446. [Google Scholar] [CrossRef]
- Smith, J.G.; Smith, A.J.; Shelton, R.M.; Cooper, P.R. Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp. Cell Res. 2012, 318, 2397–2406. [Google Scholar] [CrossRef]
- Galler, K.M.; Widbiller, M. Cell-Free Approaches for Dental Pulp Tissue Engineering. J. Endod. 2020, 46, S143–S149. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Asatourian, A.; Sorenson, C.M.; Sheibani, N. Role of angiogenesis in endodontics: Contributions of stem cells and proangiogenic and antiangiogenic factors to dental pulp regeneration. J. Endod. 2015, 41, 797–803. [Google Scholar] [CrossRef]
- Rombouts, C.; Giraud, T.; Jeanneau, C.; About, I. Pulp Vascularization during Tooth Development, Regeneration, and Therapy. J. Dent. Res. 2016, 96, 137–144. [Google Scholar] [CrossRef]
- Alghutaimel, H.; Yang, X.; Drummond, B.; Nazzal, H.; Duggal, M.; Raïf, E. Investigating the vascularization capacity of a decellularized dental pulp matrix seeded with human dental pulp stem cells: In vitro and preliminary in vivo evaluations. Int. Endod. J. 2021, 54, 1300–1316. [Google Scholar] [CrossRef]
- Suzuki, S.; Haruyama, N.; Nishimura, F.; Kulkarni, A.B. Dentin sialophosphoprotein and dentin matrix protein-1: Two highly phosphorylated proteins in mineralized tissues. Arch. Oral Biol. 2012, 57, 1165–1175. [Google Scholar] [CrossRef]
- Guo, S.; Lim, D.; Dong, Z.; Saunders, T.L.; Ma, P.X.; Marcelo, C.L.; Ritchie, H.H. Dentin Sialophosphoprotein: A Regulatory Protein for Dental Pulp Stem Cell Identity and Fate. Stem Cells Dev. 2014, 23, 2883–2894. [Google Scholar] [CrossRef]
- Martín-González, J.; Pérez-Pérez, A.; Cabanillas-Balsera, D.; Vilariño-García, T.; Sánchez-Margalet, V.; Segura-Egea, J.J. Leptin stimulates DMP-1 and DSPP expression in human dental pulp via MAPK 1/3 and PI3K signaling pathways. Arch. Oral Biol. 2019, 98, 126–131. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Z.; Liang, Y.; Xu, C.; Hong, Y.; Liu, X. Multifunctional peptide-conjugated nanocarriers for pulp regeneration in a full-length human tooth root. Acta Biomater. 2021, 127, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liang, Q.; Xu, X.; Liu, X.; Gao, X.; Li, M.; Yang, J.; Xing, X.; Huang, H.; Tang, Q.; et al. Bone morphogenetic protein 7 mediates stem cells migration and angiogenesis: Therapeutic potential for endogenous pulp regeneration. Int. J. Oral Sci. 2022, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rajani, S.; Hui, J.; Chen, A.; Bivalacqua, T.; Singh, A. Development of Enzymatic-Resistant and Compliant Decellularized Extracellular Matrixes via Aliphatic Chain Modification for Bladder Tissue Engineering. ACS Appl. Mater. Interfaces 2022, 14, 37301–37315. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fang, H.; Zheng, S.; Li, L.; Jiao, Z.; Wang, H.; Nie, Y.; Liu, T.; Song, K. A biological functional hybrid scaffold based on decellularized extracellular matrix/gelatin/chitosan with high biocompatibility and antibacterial activity for skin tissue engineering. Int. J. Biol. Macromol. 2021, 187, 840–849. [Google Scholar] [CrossRef]
- Luo, L.; He, Y.; Jin, L.; Zhang, Y.; Guastaldi, F.P.; Albashari, A.A.; Hu, F.; Wang, X.; Wang, L.; Xiao, J.; et al. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact. Mater. 2021, 6, 638–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, Y.; Wang, X.; Yuan, S.; Huo, F.; Yi, G.; Zhang, J.; Yang, B.; Tian, W. A 3D-Bioprinted Functional Module Based on Decellularized Extracellular Matrix Bioink for Periodontal Regeneration. Adv. Sci. 2022, 10, 2205041. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, Y.; Yang, X.; Chen, J.; Yang, B.; Tian, W. The Application of Pulp Tissue Derived-Exosomes in Pulp Regeneration: A Novel Cell-Homing Approach. Int. J. Nanomed. 2022, 17, 465–476. [Google Scholar] [CrossRef]
- Li, R.; Guo, W.; Yang, B.; Guo, L.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011, 32, 4525–4538. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Yang, X.; Wang, X.; Chen, J.; Tian, W.; Yang, B. Injectable Xenogeneic Dental Pulp Decellularized Extracellular Matrix Hydrogel Promotes Functional Dental Pulp Regeneration. Int. J. Mol. Sci. 2023, 24, 17483. https://doi.org/10.3390/ijms242417483
Yuan S, Yang X, Wang X, Chen J, Tian W, Yang B. Injectable Xenogeneic Dental Pulp Decellularized Extracellular Matrix Hydrogel Promotes Functional Dental Pulp Regeneration. International Journal of Molecular Sciences. 2023; 24(24):17483. https://doi.org/10.3390/ijms242417483
Chicago/Turabian StyleYuan, Shengmeng, Xueting Yang, Xiuting Wang, Jinlong Chen, Weidong Tian, and Bo Yang. 2023. "Injectable Xenogeneic Dental Pulp Decellularized Extracellular Matrix Hydrogel Promotes Functional Dental Pulp Regeneration" International Journal of Molecular Sciences 24, no. 24: 17483. https://doi.org/10.3390/ijms242417483