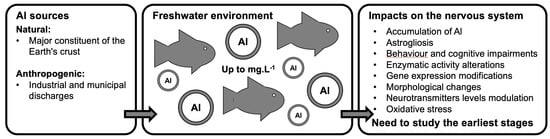
Effects of Aluminium Contamination on the Nervous System of Freshwater Aquatic Vertebrates: A Review
Abstract
:1. Introduction
2. Aluminium in Surface Freshwater
3. Effects of Aluminium on the Nervous System of Freshwater Aquatic Vertebrates
4. Perspectives: Interests of Biological Models to Study the Effects of Aluminium on the Nervous System of Aquatic Vertebrates
5. Conclusions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Monastersky, R. Biodiversity: Life—A status report. Nat. News 2014, 516, 158. [Google Scholar] [CrossRef] [PubMed]
- Beketov, M.A.; Kefford, B.J.; Schäfer, R.B.; Liess, M. Pesticides reduce regional biodiversity of stream invertebrates. Proc. Natl. Acad. Sci. USA 2013, 110, 11039–11043. [Google Scholar] [CrossRef] [Green Version]
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. Acad. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Dudley, N.; Alexander, S. Agriculture and biodiversity: A review. Biodiversity 2017, 18, 45–49. [Google Scholar] [CrossRef]
- Adjagodo, A.; Tchibozo MA, D.; Kelome, N.C.; Lawani, R. Flux des polluants liés aux activités anthropiques, risques sur les ressources en eau de surface et la chaine trophique à travers le monde: Synthèse bibliographique. Int. J. Biol. Chem. Sci. 2016, 10, 1459–1472. [Google Scholar] [CrossRef]
- Scott, G.R.; Sloman, K.A. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 2004, 68, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, S.; Mondal, P.; Nandan, A.; Siddiqui, N.A. Presence of microplastic in water bodies and its impact on human health. In Advances in Air Pollution Profiling and Control; Springer: Singapore, 2020; pp. 57–65. [Google Scholar]
- Correia, T.G.; Narcizo, A.D.M.; Bianchini, A.; Moreira, R.G. Aluminium as an endocrine disruptor in female Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 461–466. [Google Scholar] [CrossRef]
- Sfakianakis, D.G.; Renieri, E.; Kentouri, M.; Tsatsakis, A.M. Effect of heavy metals on fish larvae deformities: A review. Environ. Res. 2015, 137, 246–255. [Google Scholar] [CrossRef]
- Sparling, D.W.; Lowe, T.P. Environmental hazards of aluminium to plants, invertebrates, fish, and wildlife. Rev. Environ. Contam. Toxicol. 1996, 145, 1–127. [Google Scholar]
- Jones, K.C.; Bennett, B.G. Exposure of man to environmental aluminium—an exposure commitment assessment. Sci. Total Environ. 1986, 52, 65–82. [Google Scholar] [CrossRef]
- Ganrot, P.O. Metabolism and possible health effects of aluminium. Environ. Health Perspect. 1986, 65, 363–441. [Google Scholar]
- INERIS. Aluminium et Dérivés; Institut National de l’Environnement Industriel et des Risques—Fiche de Données Toxicologiques et Environnementales des Substances Chimiques, INERIS: Creil, France, 2005. [Google Scholar]
- Pernitsky, D.J.; Edzwald, J.K. Selection of alum and polyaluminum coagulants: Principles and applications. J. Water Supply Res. Technol. AQUA 2006, 55, 121–141. [Google Scholar] [CrossRef]
- Stahl, T.; Taschan, H.; Brunn, H. Aluminium content of selected foods and food products. Environ. Sci. Eur. 2011, 23, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Exley, C. Aluminium and medicine. Molecular and Supramolecular. Bioinorg. Chem. 2008, 6, 1–24. [Google Scholar]
- Shaman, A.M.; Kowalski, S.R. Hyperphosphatemia Management in Patients with Chronic Kidney Disease. Saudi Pharm. J. 2016, 24, 494–505. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef]
- Singla, N.; Dhawan, D.K. Zinc modulates aluminium-induced oxidative stress and cellular injury in rat brain. Metallomics 2014, 6, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef] [Green Version]
- ATSDR. Toxicological Profiles for Aluminium; US Department of Health and Human Services, Public Health Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2008.
- Turner, M.; Mutter, S.T.; Kennedy-Britten, O.D.; Platts, J.A. Molecular dynamics simulation of aluminium binding to amyloid-β and its effect on peptide structure. PLoS ONE 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Wang, L.; Hu, J.; Zhao, Y.; Lu, X.; Zhang, Q.; Niu, Q. Effects of aluminium on β-amyloid (1-42) and secretases (APP-cleaving enzymes) in rat brain. Neurochem. Res. 2014, 39, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, W.; Xu, F.; Cao, Z.; Jia, F.; Li, Y. Iron Dyshomeostasis Participated in Rat Hippocampus Toxicity Caused by Aluminum Chloride. Biol. Trace Elem. Res. 2019, 197, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Zhang, Q.; Li, H.; Wang, L.; Lu, X. The immunotoxicity and neurotoxicity of aluminium. Environ. Occup. Health Ser. 2018, 1708, 1–122. [Google Scholar]
- Hao, S.; Li, Y.F.; Hu, C.W.; Yue, S.; Li, G. Effects of sub-chronic aluminium intoxication on apoptosis of cerebrum neurocytes in chickens: Preliminary study on effect of aluminium accumulation on nervous system in chickens. In Proceedings of the International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010. [Google Scholar]
- Muhammad, M.S.; Ayo, J.O.; Danjuma, N.M.; Abdul Wahab, A.; Isa, A.S.; Maina, M.B. Molecular mechanisms of aluminium neurotoxicity in animal models of Alzheimer’s disease. J. Afr. Assoc. Physiol. Sci. 2019, 7, 70–79. [Google Scholar]
- Rosseland, B.O.; Eldhuset, T.D.; Staurnes, M.J.E.G. Environmental effects of aluminium. Environ. Geochem. Health 1990, 12, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Allin, C.J.; Wilson, R.W. Effects of pre-acclimation to aluminium on the physiology and swimming behaviour of juvenile rainbow trout (Oncorhynchus mykiss) during a pulsed exposure. Aquat. Toxicol. 2000, 51, 213–224. [Google Scholar] [CrossRef]
- Beattie, R.C.; Tyler-Jones, R. The effects of low pH and aluminium on breeding success in the frog Rana temporaria. J. Herpetol. 1992, 26, 353–360. [Google Scholar] [CrossRef]
- Bradford, D.F.; Swanson, C.; Gordon, M.S. Effects of low pH and aluminium on two declining species of amphibians in the Sierra Nevada, California. J. Herpetol. 1992, 26, 369–377. [Google Scholar] [CrossRef]
- Calevro, F.; Campani, S.; Ragghianti, M.; Bucci, S.; Mancino, G. Tests of toxicity and teratogenicity in biphasic vertebrates treated with heavy metals (Cr3+, A13+, Cd2+). Chemosphere 1998, 37, 3011–3017. [Google Scholar] [CrossRef]
- Cleveland, L.; Buckler, D.R.; Brumbaugh, W.G. Residue dynamics and effects of aluminum on growth and mortality in brook trout. Environ. Toxicol. Chem. Int. J. 1991, 10, 243–248. [Google Scholar] [CrossRef]
- Cleveland, L.; Little, E.E.; Ingersoll, C.G.; Wiedmeyer, R.H.; Hunn, J.B. Sensitivity of brook trout to low pH, low and elevated aluminum concentrations during laboratory pulse exposures. Aquat. Toxicol. 1991, 19, 303–317. [Google Scholar] [CrossRef]
- Cummins, C.P. Effects of aluminium and low pH on growth and development in Rana temporaria tadpoles. Oecologia 1986, 69, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Herkovits, J.; Castañaga, L.A.; D’Eramo, J.L.; Jourani, V.P. Living organisms influence on environmental conditions: pH modulation by amphibian embryos versus aluminum toxicity. Chemosphere 2015, 139, 210–215. [Google Scholar] [CrossRef]
- Monaco, A.; Grimaldi, M.C.; Ferrandino, I. Aluminium chloride-induced toxicity in zebrafish larvae. J. Fish Dis. 2017, 40, 629–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayer MD, J.; Reader, J.P.; Morris, R. Embryonic and larval development of brown trout, Salmo trutta L.: Exposure to aluminium, copper, lead or zinc in soft, acid water. J. Fish Biol. 1991, 38, 431–455. [Google Scholar] [CrossRef]
- Slaninova, A.; Machova, J.; Svobodova, Z. Fish kill caused by aluminium and iron contamination in a natural pond used for fish rearing: A case report. Vet. Med. 2014, 59, 573–581. [Google Scholar] [CrossRef] [Green Version]
- Stephens, F.J.; Ingram, M. Two cases of fish mortality in low pH, aluminium rich water. J. Fish Dis. 2006, 29, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Cave, M.; Zhang, C. Identifying geogenic and anthropogenic controls on different spatial distribution patterns of aluminium, calcium and lead in urban topsoil of Greater London Authority area. Chemosphere 2020, 238, 124541. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, S.J. Atmospheric input of trace metals to Lake Michigan. Water Air Soil Pollut. 1980, 13, 287–301. [Google Scholar] [CrossRef]
- His, E.; Beiras, R.; Seaman, M.N.; Pagano, G.; Trieff, N.M. Sublethal and lethal toxicity of aluminium industry effluents to early developmental stages of the Crassostrea gigas oyster. Arch. Environ. Contam. Toxicol. 1996, 30, 335–339. [Google Scholar] [CrossRef]
- Gensemer, R.W.; Playle, R.C. The Bioavailability and Toxicity of Aluminum in Aquatic Environments. Crit. Rev. Environ. Sci. Technol. 1999, 29, 315–450. [Google Scholar] [CrossRef]
- Witters, H.E. Chemical speciation dynamics and toxicity assessment in aquatic systems. Ecotoxicol. Environ. Saf. 1998, 41, 90–95. [Google Scholar] [CrossRef]
- World Health Organization. Environmental Health Criteria. 194: Aluminium. Print. Finl. 1997, 97, 1–282. [Google Scholar]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Nelson, W.O.; Campbell, P.G. The effects of acidification on the geochemistry of Al, Cd, Pb and Hg in freshwater environments: A literature review. Environ. Pollut. 1991, 71, 91–130. [Google Scholar] [CrossRef]
- Senger, M.R.; Seibt, K.J.; Ghisleni, G.C.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Aluminium exposure alters behavioral parameters and increases acetylcholinesterase activity in zebrafish (Danio rerio) brain. Cell Biol. Toxicol. 2011, 27, 199–205. [Google Scholar] [CrossRef]
- Goenaga, X.; Williams, D.J. Aluminium speciation in surface waters from a Welsh upland area. Environ. Pollut. 1988, 52, 131–149. [Google Scholar] [CrossRef]
- La Zerte, B.D.; van Loon, G.; Anderson, B. Aluminum in water. Res. Issues Alum. Toxic. 1997, 17–45. [Google Scholar]
- Huang, P.M. An overview of dynamics and biotoxicity of metals in the freshwater environment. Water Qual. Res. J. 1993, 28, 1–6. [Google Scholar] [CrossRef]
- Anandhan, R.; Hemalatha, S. Bioaccumulation of aluminium in selected tissues of Zebra fish Brachydanio rerio (Ham). Nat. Environ. Pollut. Technol. 2009, 8, 751–753. [Google Scholar]
- Exley, C. Aluminium in the brain and heart of the rainbow trout. J. Fish Biol. 1996, 48, 706–713. [Google Scholar] [CrossRef]
- Monette, M.Y.; McCormick, S.D. Impacts of short-term acid and aluminum exposure on Atlantic salmon (Salmo salar) physiology: A direct comparison of parr and smolts. Aquat. Toxicol. 2008, 86, 216–226. [Google Scholar] [CrossRef]
- Oberholster, P.J.; Myburgh, J.G.; Ashton, P.J.; Coetzee, J.J.; Botha, A.M. Bioaccumulation of aluminium and iron in the food chain of Lake Loskop, South Africa. Ecotoxicol. Environ. Saf. 2012, 75, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, S.; Khatiwada, C.P.; Sivasubramanian, J. Bioaccumulations of aluminium and the effects of chelating agents on different organs of Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2012, 34, 791–800. [Google Scholar] [CrossRef]
- Baudo, R. Sediments: Chemistry and Toxicity of In-Place Pollutants; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Capriello, T.; Grimaldi, M.C.; Cofone, R.; D’Aniello, S.; Ferrandino, I. Effects of aluminium and cadmium on hatching and swimming ability in developing zebrafish. Chemosphere 2019, 222, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, S.L.; Venkatakrishna Murali, R.; Balaji, R. Aluminium induced cholinotoxicity in zebra fish brain—A sequel of oxidative stress. Int. J. Adv. Res. 2014, 2, 322–335. [Google Scholar]
- Sukharenko, E.V.; Samoylova, I.V.; Nedzvetsky, V.S. Molecular mechanisms of aluminium ions neurotoxicity in brain cells of fish from various pelagic areas. Regul. Mech. Biosyst. 2017, 3, 461–466. [Google Scholar] [CrossRef]
- Grassie, C.; Braithwaite, V.A.; Nilsson, J.; Nilsen, T.O.; Teien, H.C.; Handeland, S.O.; Stefansson, S.O.; Tronci, V.; Gorisson, M.; Filk GEbbesson, L.O. Aluminium exposure impacts brain plasticity and behavior in Atlantic salmon (Salmo salar). J. Exp. Biol. 2013, 216, 3148–3155. [Google Scholar] [PubMed] [Green Version]
- Brodeur, J.C.; Økland, F.; Finstad, B.; Dixon, D.G.; McKinley, R.S. Effects of subchronic exposure to aluminium in acidic water on bioenergetics of Atlantic salmon (Salmo salar). Ecotoxicol. Environ. Saf. 2001, 49, 226–234. [Google Scholar] [CrossRef]
- Oliveira, V.M.; Assis, C.R.D.; Costa, H.M.S.; Silva, R.P.F.; Santos, J.F.; Carvalho, L.B., Jr.; Bezerra, R.S. Aluminium sulfate exposure: A set of effects on hydrolases from brain, muscle and digestive tract of juvenile Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 191, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Dávila, M.L.; Razo-Estrada, A.C.; García-Medina, S.; Gómez-Oliván, L.M.; Piñón-López, M.J.; Ibarra, R.G.; Galar-Martínez, M. Aluminium-induced oxidative stress and neurotoxicity in grass carp (Cyprinidae—Ctenopharingodon idella). Ecotoxicol. Environ. Saf. 2012, 76, 87–92. [Google Scholar] [CrossRef]
- Jena, B.S.; Nayak, S.B.; Patnaik, B.K. Age-related effect of aluminium on the catalase activities of the brains of two species of poikilothermic vertebrates. Gerontology 2002, 48, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Vidya, P.V.; Chitra, K.C. Aluminium oxide nanoparticles induced irrevocable damages in gill, liver and brain tissues of the freshwater Fish, Oreochromis mossambicus (Peters, 1852). Int. J. Fish. Aquat. Res. 2018, 3, 13–17. [Google Scholar]
- Laming, P.R.; Rooney, D.J.; Ferguson, J. Epileptogenesis is associated with heightened arousal responses in fish. Physiol. Behav. 1987, 40, 617–624. [Google Scholar] [CrossRef]
- Novitskiy, R.A.; Sukharenko, Y.V.; Nedzvetskiy, V.S. Molecular Biomarkers of Al 3+ Effects on Induction of Oxidative Stress and Cellular Reactivation in Organism of Lepomis gibbosus (Pisces: Centrarchidae). Hydrobiol. J. 2014, 50. [Google Scholar] [CrossRef]
- Amador, F.C.; Santos, M.S.; Oliveira, C.R. Lipid peroxidation and aluminium effects on the cholinergic system in nerve terminals. Neurotox. Res. 2001, 3, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.Y.; Guo-Ross, S.X.; Bondy, S.C. The stabilization of ferrous iron by a toxic β-amyloid fragment and by an aluminium salt. Brain Res. 1999, 839, 221–226. [Google Scholar] [CrossRef]
- Vidya, P.V.; Chitra, K.C. Aluminium oxide nanoparticles induced irreversible alterations in the antioxidant defense system of the fish, Oreochromis mossambicus (Peters, 1852). Eur. J. Biomed. Pharm. Sci. 2018, 5, 1162–1170. [Google Scholar]
- Atli, G.; Alptekin, Ö.; Tükel, S.; Canli, M. Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 218–224. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Semsei, I.; Rao, G.; Richardson, A. Expression of superoxide dismutase and catalase in rat brain as a function of age. Mech. Ageing Dev. 1991, 58, 13–19. [Google Scholar] [CrossRef]
- Sohal, R.S. Effect of hydrogen peroxide administration on life span, superoxide dismutase, catalase, and glutathione in the adult housefly, Musca domestica. Exp. Gerontol. 1988, 23, 211–216. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Vontas, J.G.; Small, G.J.; Hemingway, J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem. J. 2001, 357, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.W.; Armstrong, D. Reduced glutathione and glutathione disulfide. In Free Radical and Antioxidant Protocols; Humana Press: Totowa, NJ, USA, 1998; pp. 347–352. [Google Scholar]
- Ucán-Marín, F.; Ernst, W.; O’Dor, R.K.; Sherry, J. Effects of food borne ivermectin on juvenile Atlantic salmon (Salmo salar L.): Survival, growth, behavior, and physiology. Aquaculture 2012, 334, 169–175. [Google Scholar] [CrossRef]
- Payne, J.F.; Mathieu, A.; Melvin, W.; Fancey, L.L. Acetylcholinesterase, an old biomarker with a new future? Field trials in association with two urban rivers and a paper mill in Newfoundland. Mar. Pollut. Bull. 1996, 32, 225–231. [Google Scholar] [CrossRef]
- Gulya, K.; Rakonczay, Z.; Kasa, P. Cholinotoxic effects of aluminium in rat brain. J. Neurochem. 1990, 54, 1020–1026. [Google Scholar] [CrossRef]
- Kaizer, R.R.; Corrêa, M.C.; Spanevello, R.M.; Morsch, V.M.; Mazzanti, C.M.; Gonçalves, J.F.; Schetinger, M.R. Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminium on different mouse brain regions. J. Inorg. Biochem. 2005, 99, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Borg, D.C.; Schaich, K.M. Cytotoxicity from Coupled Redox Cycling of Autoxidizing Xenobiotics and Metals: A Selective Critical Review and Commentary on Work-in-Progress. Isr. J. Chem. 1984, 24, 38–53. [Google Scholar] [CrossRef]
- Lee, J.E.; Hollenberg, S.M.; Snider, L.; Turner, D.L.; Lipnick, N.; Weintraub, H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 1995, 268, 836–844. [Google Scholar] [CrossRef]
- Pereira, S.; Cavalie, I.; Camilleri, V.; Gilbin, R.; Adam-Guillermin, C. Comparative genotoxicity of aluminium and cadmium in embryonic zebrafish cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2013, 750, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lari, M.; Biver, T.; Busto, N.; Lozano, H.J.; Leal, J.M.; Secco, F.; García, B. Binding of Al(iii) to synthetic RNA and metal-mediated strand aggregation. Dalton Trans. 2017, 46, 16671–16681. [Google Scholar] [CrossRef]
- Wu, J.; Du, F.; Zhang, P.; Khan, I.A.; Chen, J.; Liang, Y. Thermodynamics of the interaction of aluminum ions with DNA: Implications for the biological function of aluminum. J. Inorg. Biochem. 2005, 99, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Tykhomyrov, A.A.; Pavlova, A.S.; Nedzvetsky, V.S. Glial fibrillary acidic protein (GFAP): On the 45th anniversary of its discovery. Neurophysiology 2016, 48, 54–71. [Google Scholar] [CrossRef]
- Pepeu, G.; Giovannini, M.G. Changes in acetylcholine extracellular levels during cognitive processes. Learn. Mem. 2004, 11, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Allin, C.J.; Wilson, R.W. Behavioural and metabolic effects of chronic exposure to sublethal aluminum in acidic soft water in juvenile rainbow trout (Oncorhynchus mykiss). Can. J. Fish. Aquat. Sci. 1999, 56, 670–678. [Google Scholar] [CrossRef]
- Camargo, M.M.; Fernandes, M.N.; Martinez, C.B. How aluminium exposure promotes osmoregulatory disturbances in the neotropical freshwater fish Prochilus lineatus. Aquat. Toxicol. 2009, 94, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Kim, J.W.; Yoo, D.Y.; Kim, W.; Jung, H.Y.; Choi, J.H.; Yoon, Y.S. Effects of aluminum on the reduction of neural stem cells, proliferating cells, and differentiating neuroblasts in the dentate gyrus of D-galactose-treated mice via increasing oxidative stress. J. Vet. Sci. 2016, 17, 127–136. [Google Scholar] [CrossRef]
- Wei, X.; Wei, H.; Yang, D.; Li, D.; Yang, X.; He, M.; Wu, B. Effect of aluminum exposure on glucose metabolism and its mechanism in rats. Biol. Trace Elem. Res. 2018, 186, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Andrén, C.; Henrikson, L.; Olsson, M.; Nilson, G. Effects of pH and aluminium on embryonic and early larval stages of Swedish brown frogs Rana arvalis, R. temporaria and R. dalmatina. Ecography 1988, 11, 127–135. [Google Scholar] [CrossRef]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, G.N.; Brändli, A.W. Simple vertebrate models for chemical genetics and drug discovery screens: Lessons from zebrafish and Xenopus. Dev. Dyn. 2009, 238, 1287–1308. [Google Scholar] [CrossRef]
- Slaby, S.; Marin, M.; Marchand, G.; Lemiere, S. Exposures to chemical contaminants: What can we learn from reproduction and development endpoints in the amphibian toxicology literature? Environ. Pollut. 2019, 248, 478–495. [Google Scholar] [CrossRef]
- Harland, R.M.; Grainger, R.M. Xenopus research: Metamorphosed by genetics and genomics. Trends Genet. 2011, 27, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Gurdon, J.B.; Hopwood, N. The introduction of Xenopus laevis into developmental biology: Of empire, pregnancy testing and ribosomal genes. Int. J. Dev. Biol. 2003, 44, 43–50. [Google Scholar]
- Borodinsky, L.N. Xenopus laevis as a model organism for the study of spinal cord formation, development, function and regeneration. Front. Neural Circuits 2017, 11, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, Y.; Murakami, S.; Ashikawa, Y.; Sasagawa, S.; Umemoto, N.; Shimada, Y.; Tanaka, T. Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit. Anom. 2015, 55, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Ebbert, P.T.; Lowery, L.A. Using Xenopus laevis retinal and spinal neurons to study mechanisms of axon guidance in vivo and in vitro. Semin. Cell Dev. Biol. 2016, 51, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Pratt, K.G.; Khakhalin, A.S. Modeling human neurodevelopmental disorders in the Xenopus tadpole: From mechanisms to therapeutic targets. Dis. Models Mech. 2013, 6, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Guo, S. Using zebrafish to assess the impact of drugs on neural development and function. Expert Opin. Drug Discov. 2009, 4, 715–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amora, M.; Giordani, S. The utility of zebrafish as a model for screening developmental neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Al Form | Species | Exposure Conditions | Effects | Ref. |
|---|---|---|---|---|
| AlCl3 | Danio rerio | In vivo, embryos (6 hpf) to larvae (78 hpf) Conc.: 50, 100, 200 mM (sublethal conc.) Duration: 72 h |
| [60] |
| AlCl3 | Danio rerio | In vitro, embryos (4 hpf) to larvae (48 hpf) Conc.: 100 µM 44 h |
| [37] |
| AlCl3 | Danio rerio | In vivo, adults Conc.: 150 ppm Duration: 7, 14, 21 d | In brain,
| [61] |
| AlCl3 | Danio rerio | In vivo, adults (6–8 months) Conc.: 50 µg·L−1 pH: 5.8, 6.8 Duration: 24 h (acute exposure), 96 h (chronic exposure) |
| [50] |
| AlCl3 | Danio rerio | In vitro, brain homogenate of adults (6–8 months) Conc.: 50, 100, 250 µM Duration: 10 min |
| [50] |
| AlCl3 | Danio rerio | In vivo, adults (6–8 months) Conc.: 50 µg·L1 pH: 5.8 Duration: 96 h |
| [50] |
| AlCl3 | Danio rerio | In vivo, adults Conc.: 5.69, 17.08 ppm of Al (sublethal conc.) Duration: 7, 14, 21, 28 d |
| [54] |
| AlCl3 | Carassius carassius Lepomis macrochirus Neogobius fluviatilis Rutilus rutilus | In vivo, adults (3–5 years old) Conc.: 10 mg·L−1 Duration: 45 d All 4 species were used for the experiments, except for the assessment of S100ß protein content and S100ß polypeptide fragments content in the brain (L. macrochirus and C. carassius only) |
| [62] |
| AlCl3 | Salmo salar | In vivo, pre-smolt Conc.: 0.37 ± 0.04 μmol.L−1 Al pH: 5.7 Duration: 2 weeks |
| [63] |
| AlCl3 | Channa punctatus | In vitro, brain homogenate of young, middle-aged, and old individuals Conc.: 666 µM Duration: 10 min |
| [61] |
| AlCl3 | Rana arvalis Rana temporaria Rana dalmatina | In vivo, embryonic and young larvae Conc.: 100, 200, 400, 800 μg·L−1 of Al pH: 4, 5, 6 (± 0.1) Usual pH and conc. values of acidified areas in southern Sweden Duration: until a week after hatching |
| [64] |
| Al2(SO4)3 | Oreochromis niloticus | In vivo, juveniles Conc.: 1, 3 μg·mL−1 (water treatment conc.) Duration: 14 d |
| [65] |
| Al2(SO4)3 | Oreochromis niloticus | In vitro, juveniles Conc.: 1, 3 μg·mL−1 (water treatment conc.) Duration: 1 h |
| [65] |
| Al2(SO4)3 | Ctenopharyngodon idella | In vivo, adults Conc.: 0.1 mg·L−1 of Al (maximum conc. in water to protect aquatic life; not lethal for C. idella) Duration: 12, 24, 48, 72, 96 h | In brain,
| [66] |
| Al2(SO4)3 | Cirrhinus mrigala | In vivo, adults Conc.: 5.2 (chronic exposure), 17.3 ppm (acute exposure) Duration: 15, 30, 60, 90 d (chronic exposure), 14 d (acute exposure) | In brain,
| [58] |
| Al2O3NPs | Oreochromis mossambicus | In vivo, adults (6 ± 1.5 g, 6.5 ± 1 cm) Conc.: 4 mg·L−1 (sublethal conc.) Duration: 24, 72, 96 h, and 15, 30, 60 d | In brain,
| [67] |
| Al2O3NPs | Oreochromis mossambicus | In vivo, adults (6 ± 1.5 g, 6.5 ± 1 cm) Conc.: 4 mg·L−1 (1/10th of LC50-96 h) Duration: 96 h, 60 d | In brain,
| [68] |
| Al(OH)3 | Rutilus rutilus Carassius carassius | In vivo, adults Topical application of AlOH3 gel in the midline on the surface of the posterior telencephalon in living fish Conc.: NA Duration: 6 d | In R. rutilus,
| [69] |
| Al3+ (form not specified) | Lepomis gibbosus | In vivo, adults Conc.: 10 mg·L−1 Duration: NA |
| [70] |
| Al3+ (form not specified) | Oncorhynchus mykiss | In vivo, adults (≈800 g) from an aquaculture farm in South West Scotland Conc.: 8–9 mol·L−1 (mean conc. occurring in farm water) Duration: 2 years |
| [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Closset, M.; Cailliau, K.; Slaby, S.; Marin, M. Effects of Aluminium Contamination on the Nervous System of Freshwater Aquatic Vertebrates: A Review. Int. J. Mol. Sci. 2022, 23, 31. https://doi.org/10.3390/ijms23010031
Closset M, Cailliau K, Slaby S, Marin M. Effects of Aluminium Contamination on the Nervous System of Freshwater Aquatic Vertebrates: A Review. International Journal of Molecular Sciences. 2022; 23(1):31. https://doi.org/10.3390/ijms23010031
Chicago/Turabian StyleClosset, Marie, Katia Cailliau, Sylvain Slaby, and Matthieu Marin. 2022. "Effects of Aluminium Contamination on the Nervous System of Freshwater Aquatic Vertebrates: A Review" International Journal of Molecular Sciences 23, no. 1: 31. https://doi.org/10.3390/ijms23010031