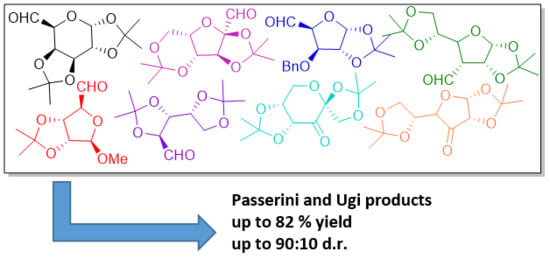
DS1,2 refers to a product isolated as an inseparable mixture of two diastereoisomers, while DS1 and DS2 refer to isolated diastereoisomer 1 and diastereoisomer 2.
3.1. General Procedure for Passerini Reactions
To a glass vial containing 1 M solution of oxo-compound (0.1 mmol) in DCM under nitrogen were added acid (0.11 mmol) and the isocyanide component (0.11 mmol) dissolved in 100 µL DCM. With all reactants added, the reaction mixture was stirred at room temperature until the consumption of the aldehyde/ketone component, which typically required 6–24 h. The reactions were concentrated under reduced pressure, and the reaction mixtures were purified by flash column chromatography.
3.1.1. Methyl 2-(2-acetoxy-2-((3aR,5aR,8aR,8bS)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4–b:4′,5′-d]pyran-3a-yl)acetamido)acetate (1c)
Yield 86% (36 mg); colorless oil; Rf (DS1,2) = 0.47 (EtOAc/petroleum ether 2:1, v/v); d.r. 90:10. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 6.95 (s, 0.9H, NH), 6.91 (s, 0.1H, NH), 5.50 (s, 0.1H, H-1 Fru), 5.18 (s, 0.9H, H-1 Fru), 4.69 (d, J = 2.6 Hz, 0.1H, H-3 Fru), 4.58 (dd, J = 7.9, 2.8 Hz, 0.9H, H-4 Fru), 4.54 (dd, J = 8.0, 2.6 Hz, 0.1H, H-4 Fru), 4.36 (d, J = 2.8 Hz, 0.9H, H-3 Fru), 4.22 (dd, J = 7.9, 1.3 Hz, 0.9H, H-5 Fru), 4.17 (dd, J = 8.0, 1.6 Hz, 0.1H, H-5 Fru), 4.09 (dd, J = 18.5, 5.0 Hz, 1H, CH2 Gly), 4.02 (dd, J = 18.5, 4.9 Hz, 1H, CH2 Gly) 3.96 (dd, J = 18.5, 4.6 Hz, 0.1H, H-6 Fru), 3.92 (dd, J = 13.0, 1.9 Hz, 0.9H, H-6 Fru), 3.83 (d, J = 13.0 Hz, 1H, H-6 Fru), 3.73 (s, 0.3H, CH3 Gly), 3.72 (s, 2.7H, CH3 Gly), 2.19 (s, 0.3H, CH3 Ac), 2.17 (s, 2.7H CH3 Ac), 1.51 (s, 3H, CH3 isop), 1.48 (s, 3H, CH3 isop), 1.44 (s, 3H, CH3 isop), 1.31 (s, 3H, CH3 isop). 13C NMR (CDCl3) δ = (170.2, 169.2, 165.7) (CO), (109.9, 109.6) (CH3 isop), (102.1, 101.5) (C-2 Fru), (75.7, 73.8, 71.6, 70.8, 70.8, 70.4, 70.3) (C-1,3,4,5 Fru), (62.0, 61.8) (C-6 Fru), (52.6, 52.4) (CH3 Gly), (41.6, 41.3) (CH2 Gly), (26.7, 26.7, 26.2, 26.0, 25.5, 25.2, 24.3, 24.2) (CH3 isop), (21.1, 20.9) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C18H27NO10Na [M + Na]+ 440.1533; found 440.1528.
3.1.2. 2-(Cyclohexylamino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4–b:4′,5′-d]pyran-5-yl)ethyl acetate (2a)
Yield 81% (35 mg); white solid mp = 111–112 °C; Rf (DS1) = 0.52, Rf (DS2) = 0.47 (EtOAc/petroleum ether 1:1, v/v); d.r. 80:20. 1H NMR (CDCl3) Chemical shifts are given for major isomer. δ = 6.18 (d, J = 7.9 Hz, 1H, NH), 5.49 (d, J = 4.8 Hz, 1H, H-1 Gal), 4.95 (d, J = 9.7 Hz, 1H, H-6 Gal), 4.60 (dd, J = 7.9, 2.6 Hz, 1H, H-3 Gal), 4.31 (dd, J = 4.8, 2.6 Hz, 1H, H-2 Gal), 4.28 (dd, J = 8.0, 1.8 Hz, 1H, H-4 Gal), 4.17 (dd, J = 9.7, 1.7 Hz, 1H, H-5 Gal), 3.84–3.70 (m, 1H, CH-1 CyHex), 2.13 (s, 3H, CH3 Ac), 1.88 (m, 2H, CyHex), 1.67 (m, 2H, CyHex), 1.56 (m, 1H, CyHex), 1.50 (s, 3H, CH3 isop), 1.41 (s, 3H, CH3 isop), 1.36–1.32 (m, 2H, CyHex), 1.31 (s, 3H, CH3 isop), 1.30 (s, 3H, CH3 isop), 1.16 (d, J = 11.9 Hz, 3H, CyHex). 13C NMR (CDCl3): δ = (169.9, 167.0) (CO), (109.8, 109.7) (C isop), 96.4 (C-1 Gal), (71.0, 70.7, 70.6, 70.6, 67.2) (C-2,3,4,5,6 Gal), 48.6 (CH CyHex), (33.04, 32.99) (CyHex), (26.29, 26.17) (CH3 isop), 25.78 (CyHex), 25.23 (CH3 isop), (24.95, 24.91) (CyHex), 24.63 (CH3 isop), 20.95 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C21H33NO8 [M + H]+ 428.2284; found 428.2291.
3.1.3. 2-(tert-Butylamino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4–b:4′,5′-d]pyran-5-yl)ethyl acetate (2b)
Yield 78% (31 mg); colorless oil; Rf (DS1) = 0.58, Rf (DS2) = 0.60 (EtOAc/petroleum ether 1:1, v/v); d.r. 80:20. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 6.19 (s, 0.8H, NH), 6.09 (s, 0.2H, NH), 5.49 (d, J = 4.8 Hz, 1H, H-1 Gal), 5.14 (d, J = 6.5 Hz, 0.2H, H-6 Gal), 4.87 (d, J = 9.8 Hz, 0.8H, H-6 Gal), 4.61–4.55 (m, 1H, H-3 Gal), 4.32–4.23 (m, 2H, H-2,4 Gal), 4.18 (d, J = 6.4 Hz, 0.2H, H-5 Gal), 4.11 (dd, J = 9.8, 1.4 Hz, 0.8H, H-5 Gal), 2.13 (s, 3H, CH3 Ac), 1.49 (s, 3H, CH3 isop), 1.40 (s, 3H, CH3 isop), 1.36–1.25 (m, 15H, CH3 isop, CH3 tBu). 13C NMR (CDCl3) δ = (170.5, 169.7, 167.2, 166.3) (CO), (109.8, 109.7, 109.7, 109.3) (C isop), (96.6, 96.4) (C-1 Gal), (73.9, 71.6, 71.1, 71.0, 70.9, 70.6, 70.6, 67.4, 67.4) (C-2,3,4,5,6 Gal), 51.7 (C tBu), (28.9, 28.7) (CH3 tBu), (26.3, 26.2, 26.2, 26.1, 25.2, 24.6, 24.3) (CH3 isop), (21.2, 20.9) (CH3 OAc). HRMS (MALDI–TOF/TOF) m/z: calcd. for C19H31NO8 [M + H]+ 402.2128; found 402.2146.
3.1.4. Methyl 2-(2-acetoxy-2-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)acetamido)acetate (2c)
Yield 78% (32 mg); colorless oil; Rf (DS1, DS2) = 0.45 (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 6.88 (s, 0.3H, NH), 6.79 (s, 0.7H, NH), 5.50 (d, J = 4.7 Hz, 1H, H-1 Gal), 5.37 (d, J = 6.8 Hz, 0.3H, H-6 Gal), 5.08 (d, J = 9.5 Hz, 0.7H, H-6 Gal), 4.61 (m, 1H, H-3 Gal), 4.41 (d, J = 8.0 Hz, 0.3H, H-4 Gal), 4.32–4.26 (m, 2H, H-4 Gal, H-2 Gal, H-5 Gal), 4.20 (dd, J = 16.5, 8.4 Hz, 1H, H-5 Gal, CH2 Gly), 4.04 (m, 1.4H, CH2 Gly), 3.91 (dd, J = 18.3, 4.3 Hz, 0.3H, CH2 Gly), 3.72 (s, 3H, CH3 Gly), 2.15 (s, 0.9H, CH3 Ac), 2.12 (s, 2.1H, CH3 Ac), 1.53 (s, 0.9H, CH3 isop), 1.51 (s, 2.1H, CH3 isop), 1.44 (s, 0.9H, CH3 isop), 1.42 (s, 2.1H, CH3 isop), 1.31 (s, 6H, CH3 isop). 13C NMR (CDCl3) δ = (170.5, 170.0, 169.9, 169.8, 168.3, 167.6) (CO), (110.0, 109.8, 109.6, 109.3) (C isop), (96.5, 96.4) (C-1 Gal), (72.9, 71.3, 71.1, 70.91, 70.8, 70.7, 70.7, 70.5, 67.2, 67.0) (C-2,3,4,5,6 Gal), (52.5, 52.5) (CH3 Gly), (41.6, 41.4) (CH2 Gly)), (26.3, 26.2, 26.1, 26.1, 25.2, 25.2, 24.7, 24.4) (CH3 isop), (21.1, 20.8) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C18H27NO10 [M + H]+ 418.1713; found 418.1711.
3.1.5. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3] dioxolo)[4,5-b:4′,5′-d] pyran-5-yl)ethyl 2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate (2d)
Yield 80% (49 mg); colorless oil; Rf (DS1,2) = 0.47 (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3) δ = 7.27–6.92 (m, 6H, Ph, NH), 5.50 (d, J = 5.0 Hz, 1.3H, H-1,6 Gal), 5.19 (d, J = 8.9 Hz, 0.7H, H-6 Gal), 4.97 (t, J = 8.0 Hz, 1H, NH), 4.67–4.45 (m, 2H, H-3 Gal, α-Phe), 4.30 (dd, J = 6.8, 4.4 Hz, 1.3H, H-2,4 Gal), 4.25–4.09 (m, 1.7H, H-5, H-4 Gal), 4.07–3.87 (m, 2H, CH2 Gly), 3.72 (d, J = 4.6 Hz, 3H, CH3 Gly), 3.22 (dd, J = 14.0, 6.2 Hz, 1H, β-Phe), 3.08 (dd, J = 14.8, 6.5 Hz, 1H, β-Phe), 1.54 (d, J = 3.8 Hz, 3H, CH3 isop), 1.46 (s, 1H, CH3 isop), 1.37 (bs, 11H, CH3 Boc, CH3 isop), 1.30 (dd, J = 8.0, 3.5 Hz, 6H, CH3 isop). 13C NMR (CDCl3) δ = (171.5, 170.8, 169.8, 169.8, 167.8, 167.3) (CO), (155.5, 155.4) (CO Boc), (136.5, 136.4) (C Ph), (129.8, 129.7, 128.7, 128.6, 127.1, 126.9) (CH Ph), (109.9, 109.8, 109.6, 109.3) (C isop), (96.4, 96.4) (C-1), (80.3, 80.1) (C Boc), (73.3, 71.6, 71.43, 71.03, 70.9, 70.6, 70.3) (C-2,3,4,6), (67.2, 67.0) (C-5 Gal), (54.9, 54.5) (α-Phe), 52.5 (CH3Gly), (41.5, 41.3) (CH2 Gly), (38.3, 37.5) (β-Phe), 28.5 (CH3 Boc), (26.3, 26.2, 26.2, 26.0, 25.2, 24.5, 24.5) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C30H42N2O12Na [M + Na]+ 645.2635; found 645.2615.
3.1.6. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3] dioxolo)[4,5-b:4′,5′-d] pyran-5-yl)ethyl 2-((tert-butoxycarbonyl)amino)-4-methylpentanoate (2e)
Yield 58% (34 mg); colorless oil; Rf (DS1,2) = 0.55, (EtOAc/petroleum ether 1:1, v/v); ¡ 75:25. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 7.33 (s, 0.25H, NH), 6.93 (s, 0.75H, NH), 5.50 (0.25, H-6 Gal), 5.50 (d, J = 4.8 Hz, 0.75H, H-1 Gal), 5.44 (d, J = 4.8 Hz, 0.25H, H-1 Gal), 5.19 (d, J = 8.9 Hz, 0.75H, H-6 Gal), 4.90 (dd, J = 16.4, 7.7 Hz, 1H, NH Leu), 4.63–4.60 (m, 0.75H, H-3 Gal), 4.57 (dd, J = 7.9, 2.1 Hz, 0.25H, H-3 Gal), 4.50 (d, J = 7.6 Hz, 0.25H, α-Leu), 4.36 (m, 0.75H, α-Leu), 4.33–4.16 (m, 3H, H-2,4,5 Gal), 4.02 (ddd, J = 41.5, 18.2, 5.3 Hz, 2H, CH2 Gly), 3.70 (d, J = 6.9 Hz, 3H, CH3 Gly), 1.74 (s, 3H, β-Leu, γ-Leu), 1.51 (d, J = 7.5 Hz, 3H, CH3 isop), 1.45–1.37 (m, 15H, CH3 Boc, CH3 isop), 1.31–1.27 (m, 3H, CH3 isop), 0.94–0.91 (m, 6H, CH3 Leu). 13C NMR (CDCl3) δ = (172.8, 172.0, 169.9, 169.8, 167.8, 167.5) (CO), (156.0, 155.7) (CO Boc), (109.9, 109.6, 109.1) (C isop), 96.4 (C-1 Gal), (80.3, 80.0) (C Boc), (72.7, 71.6, 71.0, 71.0, 70.6, 70.4) (C-2,3,4,6 Gal), (67.4, 67.0) (C-5 Gal), (52.9, 52.4, 52.3) (CH3 Gly, α-Leu), (41.8, 41.4, 41.3, 41.0) (CH2 Gly, β-Leu), 29.9, (28.5, 28.5) (Boc), (26.3, 26.2, 26.1) (CH3 isop), (25.3, 25.2) (γ-Leu), (24.9, 24.8, 24.6, 23.1, 23.0) (CH3 isop), (22.3, 22.0) (δ-Leu). HRMS (MALDI–TOF/TOF) m/z: calcd. for C27H44N2O12Na [M + Na]+ 611.2792; found 611.2805.
3.1.7. 1-tert-Butyl 2-(2-((2-methoxy-2-oxoethyl) amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3] dioxolo)[4,5-b:4′,5′-d] pyran-5-yl)ethyl)pyrrolidine-1,2-dicarboxylate (2f)
Yield 67% (40 mg); colorless oil; Rf (DS1,2) = 0.35, (EtOAc/petroleum ether 1:1, v/v); d.r. 55:45. 1H NMR (CDCl3) δ = 7.38 (dd, J = 19.5, 14.3 Hz, 0.45H, NH), 6.74 (t, J = 4.7 Hz, 0.55H, NH), 5.49 (d, J = 4.8 Hz, 1H, H-1 Gal), 5.28 (d, J = 8.0 Hz, 0.55H, H-6 Gal), 5.05 (d, J = 9.7 Hz, 0.45H, H-6 Gal), 4.60 (ddd, J = 25.8, 7.9, 2.3 Hz, 1H, H-3 Gal), 4.38–4.23 (m, 4H, H-2, H-4, H-5 Gal, CH2 Gly), 4.10 (dd, J = 17.9, 6.0 Hz, 0.55H, CH2 Gly), 4.02 (t, J = 5.7 Hz, 1H, α-Pro), 3.88 (dd, J = 17.9, 5.3 Hz, 0.45H, CH2 Gly), 3.71 (s, 1.6H, CH3 Gly), 3.68 (s, 1.4H, CH3 Gly), 3.55–3.46 (m, 1H, δ-Pro), 3.42–3.30 (m, 1H, δ-Pro), 2.33–2.26 (m, 0.55H, β-Pro), 2.22–2.13 (m, 1.5H, β-Pro), 1.88 (ddd, J = 25.6, 11.5, 8.4 Hz, 2H, γ-Pro), 1.36 (ddd, J = 36.5, 29.4, 26.3 Hz, 21H, CH3 Boc, CH3 isop). 13C NMR (CDCl3) δ = (171.8, 171.5, 169.9, 168.3, 167.8) (CO), (155.3, 154.1) (CO Boc), (109.8, 109.7, 109.6, 109.3) (C isop), (96.5, 96.4) (C-1 Gal), (80.2, 80.1) (C Boc), (71.9, 71.2, 70.9, 70.8, 70.7, 70.5, 70.4) (C-2,3,4,6 Gal), (67.1, 66.9) (C-5 Gal), (59.2, 58.9) (α-Pro), (52.4, 52.3) (CH3 Gly), (47.0, 46.5) (δ-Pro), (41.5, 41.2) (CH2 Gly), (30.9, 30.2) (β-Pro), (28.7, 28.5) (CH3 Boc), (26.3, 26.2, 26.2, 25.2, 25.2, 24.7, 24.4) (CH3 isop), (24.4, 23.4) (γ-Pro). HRMS (MALDI–TOF/TOF) m/z: calcd. for C26H40N2O12 [M + H]+ 595.2479; found 595.2453.
3.1.8. 1-Benzyl 2-tert-butyl 1-(1-(2-((2-methoxy-2-oxoethyl) amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d] pyran-5-yl)ethoxy)-1-oxo-3-phenylpropan-2-yl) hydrazine-1,2-dicarboxylate (2g)
Yield 74% (57 mg); colorless oil; Rf (DS1,2) = 0.44 (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3) δ = 7.27 (d, J = 16.6 Hz, 11H, CH Ph, NH), 5.50 (d, J = 4.8 Hz, 1H, H-1 Gal), 5.25–5.16 (m, 4H, H-6 Gal, CH2 Cbz, NH), 5.07 (m, 1H, α-hPhe), 4.55 (d, J = 10.2 Hz, 1H, H-3 Gal), 4.46 (dd, J = 8.0, 1.3 Hz, 1H, H-4 Gal), 4.30 (dd, J = 4.7, 2.5 Hz, 1H, H-2 Gal), 4.15–3.98 (m, 3H, H-5 Gal, CH2 Gly), 3.72 (s, 1.8H, CH3 Gly), 3.70 (s, 1.2H, CH3 Gly), 3.31 (dd, J = 12.8, 6.8 Hz, 1H, β-hPhe), 3.24–3.07 (m, 1H, β-hPhe), 1.54–1.12 (m, 21H, CH3 Boc, CH3 isop). 13C NMR (CDCl3) δ (169.8, 169.1, 168.5, 167.6) (CO), 155.3 (CO Boc), (137.9, 136.0) (C Ph), (129.6, 128.5, 128.4, 128.2, 127.9, 126.6) (CH Ph), (110.1, 109.8, 109.6, 109.6) (C isop), (96.4, 96.3) (C-1 Gal), (81.2, 81.2) (C Boc), (70.9, 70.8, 70.8, 70.4, 70.2) (H-2,3,4,6 Gal), (68.5, 68.3) (CH2 Cbz), (67.7, 67.1) (C-5 Gal), (62.7, 62.2) (α-Phe), 52.5 (CH3 Gly), (41.5, 41.2) (CH2 Gly), (34.6, 34.3) (β-Phe), (28.3, 28.1) (CH3 Boc), (26.2, 26.1, 26.0, 25.9, 25.1, 24.4, 24.4, 24.2) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C38H49N3O12Na [M + Na]+ 794.3112; found 794.3111.
3.1.9. 1-Benzyl 2-tert-butyl 1-(1-(2-((2-methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)ethoxy)-4-methyl-1-oxopentan-2-yl)hydrazine-1,2-dicarboxylate (2h)
Yield 53% (39 mg); colorless oil; Rf (DS1,2) = 0.54 (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3) δ = 7.31 (m, 5H, Ph Cbz), 6.91 (s, H, NH), 6.72 (s, H, NH), 5.49 (dd, J = 13.6, 4.7 Hz, 1H, H-1 Gal), 5.15 (ddd, J = 33.8, 24.8, 12.0 Hz, 4H, H-6 Gal, CH2 Cbz, NH), 4.79 (t, J = 10.2 Hz, 1H, α-hLeu), 4.63–4.48 (m, 1H, H-3 Gal), 4.39–3.86 (m, 5H, H-2,4,5 Gal, CH2 Gly), 3.72 (d, J = 3.1 Hz, 3H, CH3 Gly), 1.94 (m, 1H, β-hLeu), 1.63 (m, 1H, β-hLeu), 1.56–1.19 (m, 21H, CH3 Boc, CH3 isop), 1.06–0.80 (m, 6H, δ-hLeu). 13C NMR (CDCl3) δ = (169.8, 169.7, 167.7) (CO), (156.4, 155.1) (CO Boc), 136.1 (C Cbz), (128.8, 128.8, 128.3) (CH Cbz), (109.9, 109.86, 109.8, 109.8) (C isop), (96.4, 96.3) (C-1 Gal), (81.5, 81.4) (CO Boc), (74.4, 71.6, 71.4, 70.9, 70.6, 70.4, 70.2) (C-2,3,4,6), (68.8, 68.7) (CH2 Cbz), (67.2, 67.1, 66.9) (C-5 Gal), (60.3, 59.3) (α-Leu), 52.5 (CH3 Gly), (41.6, 39.9) (CH2 Gly), (37.0, 36.8) (β-Leu), 28.4 (CH3 Boc), (26.2, 26.2, 26.1, 26.0) (CH3 isop), (25.2, 25.2) (γ-Leu), (24.5, 24.4, 24.3, 23.9, 23.5) (CH3 isop), (21.7, 21.7) (δ-Leu).HRMS (MALDI–TOF/TOF) m/z: calcd. for C35H51N3O14 [M + Na]+ 760.3269; found 760.3243.
3.1.10. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)ethyl 1-((tert-butoxycarbonyl)amino)pyrrolidine-2-carboxylate (2i)
Yield 66% (39 mg); colorless oil; Rf (DS1,2) = 0.38 (EtOAc/petroleum ether 2:1, v/v); d.r. 70:30. 1H NMR (CDCl3) δ = 7.03 (s, 0.3H), 6.95 (s, 0.7H), 6.71 (d, J = 13.8 Hz, 1H), 5.52 (d, J = 4.8 Hz, 0.7H), 5.49 (d, J = 4.9 Hz, 0.3H), 5.37 (d, J = 8.0 Hz, 0.3H), 5.19 (d, J = 9.3 Hz, 0.7H), 4.61 (dd, J = 7.9, 2.4 Hz, 1H), 4.47 (dd, J = 8.0, 1.7 Hz, 0.3H), 4.32 (dd, J = 4.9, 2.6 Hz, 0.7H), 4.28 (dd, J = 7.8, 1.8 Hz, 1H), 4.21 (dd, J = 9.3, 1.8 Hz, 0.3H), 4.18–4.13 (m, 0.7H), 4.03 (dd, J = 7.4, 5.4 Hz, 2H), 3.92 (dd, J = 18.3, 4.8 Hz, 0.3H), 3.71 (s, 0.9H), 3.71 (s, 2.1H), 3.29 (td, J = 14.2, 7.6 Hz, 1H, δ-Pro), 3.13–3.06 (m, 1H, δ-Pro), 2.21 (dq, J = 16.4, 8.2 Hz, 1H, β-Pro), 2.13–2.03 (m, 1H, β-Pro), 1.93–1.84 (m, 2H, γ-Pro), 1.38 (m, 21H, CH3 Boc, CH3 isop). 13C NMR (CDCl3) δ = (173.0, 172.0, 170.0, 169.9, 168.3, 167.1) (CO), (110.1, 110.0, 109.7, 109.3) (C isop), (96.5, 96.4) (C-1 Gal), (80.2, 80.0) (CO Boc), (72.8, 71.1, 70.9, 70.8, 70.7, 70.6, 70.4) (C-2,3,4,6 Gal), (67.5, 67.2) (C-5 Gal), (64.8, 64.0) (α-Pro), (53.4, 53.2) (δ-Pro), (52.5, 52.4) (CH3 Gly), (41.5, 41.3) (CH2 Gly), (28.6, 28.5) (CH3 Boc), (27.9, 27.6) (β-Pro), (26.2, 26.2, 26.1, 25.2, 25.1, 24.6, 24.5) (CH3 isop), (22.3, 22.2) (γ-Pro). HRMS (MALDI–TOF/TOF) m/z: calcd. for C26H41N3O12Na [M + Na]+ 610.2588; found 610.2571.
3.1.11. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((3aR,5S,5aS,8aS,8bR)-2,2,7,7-tetramethyltetrahydro-3aH-bis([1,3]dioxolo)[4,5-b:4′,5′-d]pyran-5-yl)ethyl 2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-phenylpropanoate (2k)
Yield 74% (55 mg); colorless oil; Rf (DS1,2) = 0.39, (EtOAc/petroleum ether 1:1, v/v); d.r. 70:30. 1H NMR (CDCl3) δ = 7.73 (d, J = 7.5 Hz, 2H, Fmoc), 7.54–7.49 (m, 2H, Fmoc), 7.37 (t, J = 7.4 Hz, 2H, Fmoc), 7.30–7.19 (m, 7H, Ph), 6.96 (s, 0.3H, NH), 6.87 (s, 0.7H, NH), 5.52 (d, J = 4.7 Hz, 0.3H, H-1 Gal), 5.50 (d, J = 4.8 Hz, 0.7H, H-1 Gal), 5.44 (d, J = 7.6 Hz, 0.3H, H-6 Gal), 5.31 (d, J = 8.3 Hz, 0.7H, NH), 5.29–5.24 (m, 0.3H, NH), 5.17 (d, J = 9.2 Hz, 0.7H, H-6 Gal), 4.78–4.69 (m, 1H, α-Phe), 4.58 (ddd, J = 32.1, 7.8, 2.2 Hz, 1H, H-3 Gal), 4.38 (dt, J = 18.2, 9.1 Hz, 1H, CH2 Fmoc), 4.30 (dd, J = 4.9, 2.6 Hz, 1H, H-2 Gal), 4.27–4.00 (m, 6H, CH2 Gly, CH Fmoc, CH2 Fmoc, H-5,4 Gal), 3.72 (s, 2.1H, CH3 Gly), 3.68 (s, 0.9H, CH3 Gly), 3.26 (dd, J = 14.0, 6.2 Hz, 1H, β-Phe), 3.13 (dd, J = 14.0, 6.4 Hz, 1H, β-Phe), 1.55–1.21 (m, 12H, CH3 isop). 13C NMR (CDCl3) δ (171.1, 170.5, 169.8, 167.7) (CO Fmoc), (155.9, 155.8) (CO Fmoc), (144.0, 143.9, 141.5) (C Fmoc), (136.4, 136.1) (C Ph), (129.8, 129.8, 128.8, 128.6, 127.9, 127.2, 127.1) (CH Ph), (125.3, 125.3, 125.2) (CH Fmoc-Ph), (120.2, 120.1) (CH Fmoc-Ph), (110.0, 109.9, 109.6, 109.3) (C isop), (96.4, 96.4) (C-1 Gal), (73.5, 71.5, 71.3, 71.0, 70.9, 70.8, 70.5, 70.3) (C-2,3,4,6 Gal), 67.4 (C-5 Gal), 67.2 (CH2 Fmoc), 67.0 (C-5 Gal), (55.1, 54.9) (α-Phe), (52.5, 52.4) (CH3 Gly), (47.4, 47.3) (CH Fmoc), (41.5, 41.3) (CH2 Gly), (38.3, 37.6) (β-Phe), (26.3, 26.2, 26.2, 26.0, 25.2, 24.5, 24.4) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C40H44N2O12 [M + H]+ 745.2972; found 745.2990.
3.1.12. 2-(Cyclohexylamino)-1-((3aR,4S,6R,6aR)-6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3] dioxol-4-yl)-2-oxoethyl acetate (3a)
Yield 82% (30 mg); colorless oil; Rf = 0.63 (EtOAc/petroleum ether 1:1, v/v); d.r. 77:23. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 5.97 (d, J = 7.5 Hz, 0.77H, NH), 5.90 (d, J = 7.7 Hz, 0.23H, NH), 5.11 (d, J = 7.6 Hz, 0.23H, H-5 Rib), 5.07 (d, J = 6.9 Hz, 0.77H, H-5 Rib), 4.95 (s, 0.23H, H-1 Rib), 4.94 (s, 0.77H, H-1 Rib), 4.87 (dd, J = 6.0, 1.4 Hz, 0.23H, H-2 Rib), 4.71 (dd, J = 6.2, 0.5 Hz, 0.77H, H-2 Rib), 4.60 (d, J = 6.9 Hz, 0.77H, H-4 Rib), 4.55 (d, J = 6.0 Hz, 0.77H, H-3 Rib), 4.53 (d, J = 6.0 Hz, 0.23H, H-3 Rib), 4.50 (dd, J = 7.6, 1.4 Hz, 0.23H, H-4 Rib), 3.79–3.72 (m, 1H, CH-1 CyHex), 3.32 (s, 2.3H, OCH3), 3.28 (s, 0.7H, OCH3), 2.15 (s, 0.7H, CH3 Ac), 2.13 (s, 2.3H, CH3 Ac), 1.89 (m, 2H, CyHex), 1.66 (m, 2H, CyHex), 1.58 (m, 1H, CyHex), 1.45 (s, 3H, CH3 isop), 1.34 (m, 2H, CyHex), 1.29 (s, 2.3H, CH3 isop), 1.28 (s, 0.7H, CH3 isop), 1.16–1.14 (m, 3H, CyHex). 13C NMR (CDCl3) δ = (170.0, 166.1) (CO), 112.9 (C isop), (111.1, 110.3) (C-1 Rib), (86.2, 86.0) (C-4 Rib), (85.4, 85.3) (C-3 Rib), (81.7, 81.4) (C-2 Rib), (74.8, 73.6) (C-5 Rib), (56.2, 55.5) (OCH3), 48.5 (CH CyHex), 48.35 (CH CyHex), (33.1, 33.1) (CyHex), (26.8, 26.7) (CH3 isop), (25.7, 25.6, 25.3, 24.9, 24.9) (CyHex), (21.4, 21.1) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C18H29NO7Na [M + Na]+ 394.1842; found 394.1843.
3.1.13. 1-((3aR,4S,6R,6aR)-6-Methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-2-((2-methoxy-2-oxoethyl)amino)-2-oxoethyl 2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate (3d)
Yield 78% (44 mg); colorless oil; Rf (DS1) = 0.53, (EtOAc/petroleum ether 1:1, v/v); d.r. 77:23. 1H NMR (CDCl3) δ = 7.36–7.08 (m, 5H, CH Ph), 7.15 (s, 1H, NH), 5.36 (d, J = 4.6 Hz, 1H, NH), 5.19 (d, J = 4.8 Hz, 0.23H, H-5 Rib), 5.04 (d, J = 7.6 Hz, 0.77H, H-5 Rib), 4.96 (s, 0.77H, H-1 Rib), 4.94 (s, 0.23H, H-1 Rib), 4.74–4.53 (m, 4H, H-2,3,4 Rib, α-Phe), 4.09 (d, J = 5.8 Hz, 0.23H, CH2 Gly), 4.03 (d, J = 5.8 Hz, 0.77H, CH2 Gly), 3.94 (d, J = 5.4 Hz, 0.77H, CH2 Gly), 3.88 (d, J = 5.4 Hz, 0.23H, CH2 Gly), 3.71 (d, J = 5.2 Hz, 3H, CH3 Gly), 3.33–3.30 (m, 3.77H, CH3 Rib, β-Phe), 2.98 (dd, J = 14.3, 8.8 Hz, 1.23H, β-Phe), 1.46 (s, 2.31H, CH3 isop), 1.42 (s, 0.69H, CH3 isop), 1.39 (s, 2.31H, Boc), 1.37 (s, 6.9H, Boc), 1.31 (s, 2.1H, CH3 isop), 1.27 (s, 0.69H, CH3 isop). 13C NMR (CDCl3) δ = (170.5, 169.6, 167.2) (CO), 156.2 (CO Boc), 136.4 (C Ph), (129.6, 129.3, 129.0, 128.9, 127.6, 127.3) (CH Ph), 112.8 (C isop), (111.9, 109.9) (C-1 Rib), (86.4, 86.2) (C-4 Rib), (85.7, 85.6) (C-3 Rib), (81.2, 81.0) (C-2 Rib), (80.9, 80.7) (C Boc), (75.0, 74.5) (C-5 Rib), (56.3, 55.9) (α-Phe), (55.3, 54.9) (CH3 Rib), (52.5, 52.3) (CH3 Gly), (41.2, 41.1) (CH2 Gly), (37.6, 37.6) (β-Phe), (28.5, 28.4) (CH3 Boc), (26.9, 26.7) (CH3 isop), (25.7, 25.3) CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C27H38N2O11Na [M + Na]+ 589.2373; found 589.2399.
3.1.14. 1-((3aR,5S,6S,6aR)-6-(Benzyloxy)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-5-yl)-2-(cyclohexylamino)-2-oxoethyl acetate (4a)
Yield 79% (36 mg); colorless oil; Rf (DS1) = 0.40, Rf (DS2) = 0.26 (EtOAc/petroleum ether 1:2, v/v); d.r. 77:23. 1H NMR (CDCl3) Chemical shifts are given for major isomer. δ = 7.41–7.14 (m, 5H, Ph), 6.01 (d, J = 7.9 Hz, 1H, NH), 5.92 (d, J = 3.7 Hz, 1H, H-1 Xyl), 5.11 (d, J = 9.2 Hz, 1H, H-5 Xyl), 4.62 (d, J = 11.6 Hz, 1H, CH2a-Ph), 4.60 (d, J = 3.7 Hz, 1H, H-2 Xyl), 4.45–4.38 (m, 2H, H-4 Xyl, CH2b-Ph), 3.99 (d, J = 3.1 Hz, 1H, H-3 Xyl), 3.79–3.70 (m, 1H, CH-1 CyHex), 1.98 (s, 3H, CH3 Ac), 1.84 (m, 2H, CyHex), 1.63 (m, 2H), 1.55–1.50 (m, 1H, CyHex), 1.45 (s, 3H, CH3 isop), 1.30 (bs, 5H, CH3 isop, CyHex), 1.16–1.06 (m, 3H, CyHex). 13C NMR (CDCl3) δ (169.70, 166.78) (CO), 136.97 (C Ph), (128.84, 128.45, 128.35) (CH Ph), 112.61 (C isop), 105.65 (C-1 Xyl), (81.79, 81.30, 78.64, 72.47, 70.22) (C-2,3,4,5-Xyl, CH2-Ph), 48.52 (CH CyHex), (32.89, 32.83) (CyHex), (27.01, 26.58) (CH3 isop), (25.73, 24.79, 24.75) (CyHex), 20.78 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C24H33NO7 [M + H]+ 448.2335; found 448.2346.
3.1.15. 1-((3aR,5S,6S,6aR)-6-(Benzyloxy)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-5-yl)-2-((2-methoxy-2-oxoethyl) amino)-2-oxoethyl 2-((tert-butoxycarbonyl) amino)-3-phenylpropanoate (4d)
Yield 75% (48 mg); colorless oil; Rf (DS1) = 0.52, (EtOAc/petroleum ether 1:1, v/v); d.r. 77:23. 1H NMR (CDCl3) δ = 7.42–7.11 (m, 10H, Bn, Ph), 6.86 (s, 1H, NH), 5.94 (d, J = 3.6 Hz, 1H, H-1 Xyl), 5.65 (d, J = 7.2 Hz, 0.23H, H-5 Xyl), 5.43 (d, J = 9.0 Hz, 0.77H, H-5 Xyl), 4.91 (m, 1H, α-Phe), 4.67–4.39 (m, 5.77H, H-2, H-4, H-3 Xyl, NH, CH2 OBn), 4.24 (d, J = 3.9 Hz, 0.23H, H-3 Xyl), 4.02 (d, J = 5.3 Hz, 0.77H, CH2 Gly), 3.99 (m, 0.77H, CH2 Gly), 3.92 (d, J = 5.7 Hz, 0.46H, CH2 Gly), 3.71 (s, 2.31H, CH3 Gly), 3.69 (s, 0.69H, CH3 Gly), 3.28 (dd, J = 14.1, 5.6 Hz, 0.77H, β-Phe), 3.12 (dd, J = 14.2, 5.3 Hz, 0.23H, β-Phe), 3.00 (dd, J = 14.1, 7.7 Hz, 0.77H, β-Phe), 2.88 (dd, J = 14.1, 8.3 Hz, 0.23H, β-Phe), 1.48 (s, 3H, CH3 isop), 1.35 (s, 3H, CH3 isop), 1.30 (s, 9H, CH3 Boc). 13C NMR (CDCl3) δ (171.0, 169.8, 167.7) (CO), 155.4 (CO Boc), (137.1, 136.5, 136.1) (C Ph), (129.7, 129.5, 128.8, 128.7, 128.6, 128.4, 128.3, 128.2, 127.2, 127.0) (CH Ph), (112.8, 112.4) (C isop), (105.8, 105.2) (C-1 Xyl), (83.0, 83.0) (C-2 Xyl), (81.9, 81.4) (C-3 Xyl), (80.6, 80.0) (C Boc), (79.0, 78.5) (C-4 Xyl), (72.8, 72.6) (CH2-OBn), (72.1, 70.0) (C-5 Xyl), (55.0, 54.3) (α-Phe), (52.5, 52.4) (CH3 Gly), (41.5, 41.1) (CH2 Gly), (37.9, 37.1) (β-Phe), 28.3 (CH3 Boc), (28.35, 27.2, 27.0, 26.6, 26.5) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C33H42N2O11Na [M + Na]+ 665.2686; found 665.2682.
3.1.16. 2-(Cyclohexylamino)-2-oxo-1-((3aS,3bR,7aS,8aR)-2,2,5,5-tetramethyltetrahydro-3aH-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxin-8a-yl)ethyl acetate (5a)
Yield 66% (28 mg); colorless oil; Rf (DS1) = 0.50, Rf (DS2) = 0.40 (toluen/aceton 5:1, v/v); d.r. 90:10. 1H NMR (CDCl3) Chemical shifts are given for major isomer. δ = 6.30 (d, J = 7.6 Hz, 1H, NH), 5.26 (s, 1H, H-1 Sor), 4.41 (s, 1H, H-5 Sor), 4.31 (d, J = 2.2 Hz, 1H, H-4 Sor), 4.17 (d, J = 1.5 Hz, 1H, H-3 Sor), 4.07 (dd, J = 13.6, 2.2 Hz, 1H, H-6a Sor), 3.98 (d, J = 13.5 Hz, 1H, H-6b Sor), 3.85–3.75 (m, 1H, CH-1 CyHex), 2.17 (s, 3H, CH3 Ac), 1.89 (m, 2H, CyHex), 1.63 (s, 3H, CyHex), 1.54 (m, 1H, CyHex), 1.46 (s, 3H, CH3 isop), 1.41 (d, 6H, CH3 isop), 1.38 (bs, 3H, CH3 isop), 1.34 (m, 1H, CyHex), 1.24–1.17 (m, 3H, CyHex). 13C NMR (CDCl3): δ = (169.6, 164.6) (CO), (113.8, 113.2) (C isop), 97.8 (C-2 Sor), 85.66 (C-5 Sor), 73.7 (C-3 Sor), 72.9 (C-4 Sor), 72.7 (C-1 Sor), 60.6 (C-6 Sor), 48.4 (CH CyHex), 32.9 (CyHex), (32.8, 29.2) (CyHex), (27.8, 26.5, 25.9) (CH3 isop), (24.7, 24.7) (CyHex), 21.0 (CH3 isop), 18.9 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C21H33NO8 [M + H]+ 428.2284; found 428.2272.
3.1.17. Methyl 2-(2-acetoxy-2-((3aS,3bR,7aS,8aR)-2,2,5,5-tetramethyltetrahydro-3aH-[1,3]dioxolo[4′,5′:4,5]furo [3,2-d][1,3] dioxin-8a-yl)acetamido)acetate (5c)
Yield 63% (26 mg); colorless oil; Rf (DS1) = 0.30, Rf (DS2) = 0.27 (EtOAc/petroleum ether 1:1, v/v); d.r. 87:13. 1H NMR (CDCl3) δ = 7.10 (s, 0.87H, NH), 6.84 (s, 0.13H, NH), 5.68 (s, 0.13H, H-1 Sor), 5.39 (s, 0.87H, H-1 Sor), 4.87 (s, 0.13H, H-5 Sor), 4.41 (s, 0.87H, H-5 Sor), 4.33 (d, J = 1.7 Hz, 0.87H, H-4 Sor), 4.29 (d, J = 2.3 Hz, 0.13H, H-4 Sor), 4.23–3.96 (m, 5H, H-3,6 Sor, CH2 Gly), 3.73 (d, J = 1.8 Hz, 3H, CH3 Gly), 2.22 (s, 0.39H, CH3 Ac), 2.18 (s, 2.61H, CH3 Ac), 1.47–1.30 (m, 12H, CH3 isop). 13C NMR (CDCl3) δ (170.2, 169.4, 165.6) (CO), 114.0 (C isop), 112.9 (C isop), 97.8 (C-2 Sor), 85.6 (C-5 Sor), (74.5, 74.0, 73.5, 73.2, 72.8, 72.4) (C-1,3,4 Sor), 60.4 (C-6 Sor), 52.5 (CH3 Gly), (41.7, 41.3) (CH2 Gly), (29.1, 28.5, 27.9, 27.7, 26.4) (CH3 isop), (19.2, 18.8) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C18H27NO10 [M + H]+ 418.1713; found 418.1697.
3.1.18. 2-((2-Methoxy-2-oxoethyl) amino)-2-oxo-1-((3aS,3bR,7aS,8aR)-2,2,5,5-tetramethyltetrahydro-3aH-[1,3]dioxolo[4′,5′:4,5]furo[3,2-d][1,3]dioxin-8a-yl)ethyl 2-((tert-butoxycarbonyl) amino)-3-phenylpropanoate (5d)
Yield 65% (40 mg); colorless oil; Rf (DS1,2) = 0.34 (EtOAc/petroleum ether 1:1, v/v); d.r. 90:10. 1H NMR (CDCl3) δ = 7.35–7.05 (m, 6H, CH Ph, NH), 5.50 (s, 1H, H-1 Sor), 4.94 (d, J = 8.4 Hz, 1H, NH Phe), 4.65 (dd, J = 12.9, 8.0 Hz, 1H, α-Phe), 4.42 (s, 1H, H-5 Sor), 4.36 (s, 1H, H-4 Sor), 4.25 (s, 1H, H-3 Sor), 4.17 (dd, J = 18.5, 4.9 Hz, 1H, CH2 Gly), 4.14–4.10 (m, 2H, H-6 Sor), 4.07 (dd, J = 18.5, 4.7 Hz, 1H, CH2 Gly), 3.76 (s, 3H, CH3 Gly), 3.42 (dd, J = 14.2, 4.7 Hz, 1H, β-Phe), 3.06 (dd, J = 14.2, 8.2 Hz, 1H, β-Phe), (1.51, 1.44, 1.40, 1.36) (21H, CH3 isop, CH3 Boc). 13C NMR (CDCl3) δ (170.8, 170.1, 165.3) (CO), 155.4 (CO Boc), 136.6 (C Ph), (129.8, 128.7, 126.9) (CH Ph), (114.1, 112.8) (C isop), 97.8 (C-2 Sor), 85.5 (C-5 Sor), 80.0 (C Boc), 74.2 (C-3 Sor), 72.7 (C-4 Sor), 60.5 (C-6 Sor), 54.6 (α-Phe), 52.5 (CH3 Gly), 41.8 (CH2 Gly), 38.4 (β-Phe), (29.1, 28.4, 27.7, 26.5) (CH3 isop), 18.9 (CH3 Boc). HRMS (MALDI–TOF/TOF) m/z: calcd. for C30H42NO12Na [M + Na]+ 645.2635; found 645.2641.
3.1.19. 2-(Cyclohexylamino)-1-((3aR,5S,6R,6aR)-5-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl)-2-oxoethyl acetate (6a)
Yield 72% (32 mg); colorless oil; Rf (DS1, DS2) = 0.37, (EtOAc/petroleum ether 1:1, v/v); d.r. 90:10. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 6.04 (d, J = 7.9 Hz, 1H, NH), 5.75 (d, J = 3.6 Hz, 0.9H, H-3’ Alo), 5.73 (d, J = 3.7 Hz, 0.1H, H-3’ Alo), 5.37 (d, J = 6.4 Hz, 0.1H, H-1 Alo), 5.21 (d, J = 8.7 Hz, 0.9H, H-1 Alo), 4.69–4.62 (m, 1H, H-2 Alo), 4.27–4.16 (m, 2H, H-4,5 Alo), 4.03 (dd, J = 8.4, 6.5 Hz, 1H, H-6 Alo), 3.95 (dd, J = 8.4, 6.6 Hz, 0.9H, H-6 Alo), 3.88 (dd, J = 8.1, 5.4 Hz, 0.1H, H-6 Alo), 3.76 (m, 1H, CH-1 CyHex), 2.51 (m, 0.1H, H-3 Alo), 2.40–2.28 (m, 0.9H, H-3 Alo), 2.11 (s, 2.7H, CH3 Ac), 2.09 (s, 0.3H, CH3 Ac), 1.86 (s, 2H, CyHex), 1.70–1.54 (m, 3H, CyHex), 1.50 (s, 3H, CH3 isop), 1.40 (s, 3H, CH3 isop), 1.38–1.33 (m, 2H, CyHex), 1.32 (s, 3H, CH3 isop), 1.27 (s, 3H, CH3 isop), 1.20–1.11 (m, 3H, CyHex). 13C NMR (CDCl3) δ (170.2, 167.2) (CO), (112.7, 109.8) (C isop), (105.1, 104.6) (C-1 Alo), (81.6, 81.4, 80.3, 78.5, 76.9, 71.4, 71.3) (C-2,3’,4,5), (66.9, 65.8) (C-6 Alo), (48.6) (C-3 Alo), (48.4, 48.3) (CH CyHex), 32.9 (CyHex), (27.0, 26.6, 26.5, 25.7) (CH3 isop), (25.7, 24.8) (CyHex), 21.1 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C22H35NO8Na [M + Na]+ 464.2260; found 464.2254.
3.1.20. Methyl 2-(2-acetoxy-2-((3aS,5S,6R,6aS)-5-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl)acetamido) acetate (6c)
Yield 74% (32 mg); colorless oil; Rf (DS1, DS2) = 0.38, (EtOAc/petroleum ether 1:1, v/v); d.r. 90:10. 1H NMR (CDCl3) Chemical shifts are given for both isomers. δ = 6.77 (s, 0.9H, NH), 6.74 (s, 0.1H, NH), 5.76 (d, J = 3.6 Hz, 0.9H, H-3’ Alo), 5.73 (d, J = 3.7 Hz, 0.1H, H-3’ Alo), 5.48 (d, J = 6.0 Hz, 0.1H, H-1 Alo), 5.38 (d, J = 8.2 Hz, 0.9H, H-1 Alo), 4.82 (t, J = 4.1 Hz, 0.9H, H-2 Alo), 4.77 (t, J = 4.1 Hz, 0.1H, H-2 Alo), 4.26 (dd, J = 9.7, 5.0 Hz, 0.9H, H-4 Alo), 4.22–4.06 (m, 2.1H, H-4,5 Alo, CH2 Gly), 4.05–4.00 (m, 1H, H-6 Alo), 3.94 (dd, J = 8.2, 6.9 Hz, 0.9H, H-6 Alo), 3.87 (dd, J = 18.3, 4.4 Hz, 1.1H, H-6 Alo, CH2 Gly), 3.74 (s, 0.3H, CH3 Gly), 3.72 (s, 2.7H, CH3 Gly), 2.52–2.45 (m, 0.1H, H-3 Alo), 2.37–2.30 (m, 0.9H, H-3 Alo), 2.12 (s, 2.7H, CH3 Ac), 2.10 (s, 0.3H, CH3 Gly), 1.77 (s, 0.3H, CH3 isop), 1.50 (s, 0.3H, CH3 isop), 1.47 (s, 2.7H, CH3 isop), 1.39 (s, 2.7H, CH3 isop), 1.30 (s, 3H, CH3 isop), 1.28 (s, 3H, CH3 isop). 13C NMR (CDCl3) δ (170.1, 170.0, 168.8) (CO), (112.7, 109.8) (C isop), (105.1, 104.6) (C-1 Alo), (81.3, 81.2, 79.9, 78.4, 77.3, 76.9, 71.0, 70.7) (C-2,3’,4,5), (67.2, 66.0) (C-6 Alo), (52.7, 52.5) (CH3 Gly), (48.8, 48.7, 48.3) (CH CyHex, C-3 Alo)), (41.3, 41.2) (CH2 Gly), (26.9, 26.8, 26.7, 26.5, 26.4, 25.6, 25.2) (CH3 isop), (21.0, 20.9) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C19H29NO10Na [M + Na]+ 454.1689; found 454.1696.
3.1.21. 1-((3aS,6aS)-5-(2,2-Dimethyl-1,3-dioxolan-4-yl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl)-2-((2-methoxy-2-oxoethyl)amino)-2-oxoethyl 2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate (6d)
Yield 77% (49 mg); colorless oil; Rf (DS1,2) = 0.47 (EtOAc/petroleum ether 1:1, v/v); d.r. 90:10. 1H NMR (CDCl3) δ 7.33–7.16 (m, 5H, Ph), 6.92 (s, 1H, NH), 5.75 (d, J = 3.7 Hz, 1H, H-3’ Alo), 5.49 (d, J = 7.6 Hz, 1H, H-1 Alo), 5.01 (d, J = 7.4 Hz, 1H, NH Phe), 4.78 (t, J = 4.0 Hz, 1H, α-Phe), 4.59 (dd, J = 13.3, 6.7 Hz, 1H, H-2 Alo), 43.1 (dd, J = 9.7, 4.4 Hz, 1H, H-5 Alo), 4.12 (ddd, J = 22.6, 11.3, 5.5 Hz, 2H, H-4 Alo, CH2 Gly), 3.98 (d, J = 6.5 Hz, 2H, H-6 Alo), 3.86 (dd, J = 18.0, 4.9 Hz, 1H, CH2 Gly), 3.72 (s, 3H, CH3 Gly), 3.22 (dd, J = 14.1, 5.7 Hz, 1H, β-Phe), 3.07 (dd, J = 14.0, 7.1 Hz, 1H, β-Phe), 2.35 (ddd, J = 9.9, 7.8, 4.6 Hz, 1H, H-3 Alo), 1.57–1.28 (m, 21H, CH3 Boc, CH3 isop). 13C NMR (CDCl3) δ (171.1, 169.9, 168.6) (CO), 155.7 (CO Boc), 136.2 (C Ph), (129.6, 128.8, 127.3) (CH Ph), (112.7, 110.0) (C isop), 104.6 (C-1 Alo), 81.3 (C-2 Alo), 80.5 (C Boc), 79.5 (C-4 Alo), 76.6 (C-5 Alo), 71.3 (C-3’ Alo), 65.8 (C-6 Alo), 54.8 (α-Phe), 52.5 (CH3 Gly), 48.2 (C-3 Alo), 41.3 (CH2 Gly), 37.9 (β-Phe), 28.49 (CH3 Boc), (26.86, 26.62, 26.39, 25.44) (CH3 isop).HRMS (MALDI–TOF/TOF) m/z: calcd. for C31H44N2O12Na [M + Na]+ 659,2792; found 659,2790.
3.1.22. 2-(Cyclohexylamino)-2-oxo-1-((4R,4’R,5S)-2,2,2’,2’-tetramethyl-[4,4’-bi(1,3-dioxolan)]-5-yl)ethyl acetate (7a)
Yield 81% (32 mg); colorless oil; Rf (DS1) = 0.50, Rf (DS2) = 0.48 (EtOAc/petroleum ether 1:1, v/v); d.r. 55:45. 1H NMR (CDCl3) δ = 5.95 (d, J = 7.9 Hz, 0.55H, NH), 5.77 (d, J = 8.1 Hz, 0.45H, NH), 5.37 (d, J = 2.7 Hz, 0.55H, H-1 Ara), 5.28 (d, J = 2.3 Hz, 0.45H, H-1 Ara), 4.37 (dd, J = 7.5, 2.3 Hz, 0.45H, H-2 Ara), 4.32 (t, J = 7.8 Hz, 0.55H, H-3 Ara), 4.10 (dd, J = 8.0, 2.7 Hz, 0.55H, H-2 Ara), 4.08–3.99 (m, 2H, H-4, H-5 Ara), 3.94 (dd, J = 8.4, 4.9 Hz, 0.55H, H-5 Ara), 3.89 (m, 0.45H, H-5 Ara), 3.78–3.70 (m, 1.45H, H-3 Ara, CH-1 CyHex), 2.15 (s, 1.35H, CH3 Ac), 2.14 (s, 1.65H, CH3 Ac), 1.85 (m, 2H, CyHex), 1.64 (m, 2H, CyHex), 1.56 (m, 1H, CyHex), 1.45 (s, 2H, CH3 isop), 1.36–1.26 (m, 12H, CH3 isop, CyHex), 1.17–1.07 (m, 3H, CyHex). 13C NMR (CDCl3) δ = (169.3, 169.13, 166.7, 165.9) (CO), (110.5, 110.2, 110.2, 110.1) (C isop), (80.1, 79.4) (C-2 Ara), (77.4, 77.2) (C-3 Ara), (77.1, 76.9) (C-4 Ara), (73.7, 72.7) (C-1 Ara), 67.4 (C-5 Ara), (48.4, 48.3) (CH CyHex), (33.2, 33.1, 33.1, 33.0) (CyHex), (27.4, 27.4, 27.0, 27.0, 26.7) (CH3 isop), (25.7, 25.7) (CyHex), (25.6, 25.3) (CH3 isop), (24.9, 24.8) (CyHex), (21.1, 21.0) (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C20H33NO7Na [M + Na]+ 422.2155; found 422.2138.
3.1.23. 2-((2-Methoxy-2-oxoethyl)amino)-2-oxo-1-((4R,4’R,5S)-2,2,2’,2’-tetramethyl-[4,4’-bi(1,3-dioxolan)]-5-yl)ethyl 2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate (7d)
Yield 78% (46 mg); colorless oil; Rf (DS1) = 0.48 (EtOAc/petroleum ether 1:1, v/v); d.r. 55:45. 1H NMR (CDCl3) δ 7.60 (m, 0.55H, NH), 7.36–7.25 (m, 5H, CH Ph), 7.11 (m, 0.45H, NH), 5.50 (d, J = 2.7 Hz, 0.55H, H-1 Ara), 5.44 (d, J = 1.6 Hz, 0.45H, H-1 Ara), 5.12 (d, J = 6.0 Hz, 0.45H, α-Phe), 4.97 (d, J = 7.1 Hz, 0.55H, α-Phe), 4.65–4.45 (m, 1.45H, H-2 Ara, NH), 4.29 (t, J = 7.4 Hz, 0.55H, H-3 Ara), 4.15–3.92 (m, 5.45H, H-2, H-3, H-4, H-5, CH2 Gly), 3.81 (td, J = 7.9, 3.4 Hz, 0.55H, H-5 Ara), 3.73 (s, 1.65H, CH3 Gly), 3.71 (s, 1.35H, CH3 Gly), 3.21–3.05 (m, 2H, β-Phe), 1.47–1.27 (m, 21H, CH3 Boc, CH3 isop). 13C NMR (CDCl3) δ (171.7, 170.6, 169.8, 169.6, 168.0, 167.0) (CO), 156.0 (CO Boc), (135.9, 135.5) (C Ph), (129.4, 129.3, 129.2, 129.0, 127.6, 127.4) (CH Ph), (110.5, 110.3, 110.0, 109.9) (C isop), (81.0, 80.8) (C Boc), (79.6, 79.2) (C-2 Ara), 77.1 (C-3 Ara), (76.8, 76.6) (C-4 Ara), (74.2, 72.9) (C-1 Ara), (67.47, 67.2) (C-5 Ara), (55.9, 54.8) (α-Phe), (52.4, 52.3) (CH3 Gly), (41.3, 41.1) (CH2 Gly), (37.8, 37.7) (β-Phe), (28.4, 28.4) (CH3 Boc), (27.4, 27.4, 26.9, 26.9, 26.7, 26.6, 25.5, 25.1) (CH3 isop). HRMS (MALDI–TOF/TOF) m/z: calcd. for C29H42N2O11Na [M + Na]+ 617.2686; found 617.2687.
3.1.24. (3aR,5S,6aR)-6-(Cyclohexylcarbamoyl)-5-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl acetate (8a)
Yield 67% (29 mg); colorless oil; Rf (DS1) = 0.70, Rf (DS2) = 0.50 (EtOAc/petroleum ether 1:1, v/v); d.r. 55:45. 1H NMR (CDCl3) Major isomer: δ = 7.40 (d, J = 7.5 Hz, 1H, NH), 5.88 (d, J = 3.6 Hz, 1H, H-1 GlcF), 4.87 (d, J = 3.6 Hz, 1H, H-2, GlcF), 4.45 (t, J = 4.7 Hz, 1H, H-5, GlcF), 4.42 (d, J = 2.8 Hz, 1H, H-4 GlcF), 4.13 (dd, J = 8.4, 6.5 Hz, 1H, H-6a GlcF), 4.06 (dd, J = 8.4, 6.9 Hz, 1H, H-6b, GlcF), 3.80–3.73 (m, 1H, CH-1 CyHex), 2.09 (m, 3H, CH3 Ac), 1.93–1.82 (m, 2H, CyHex), 1.67–1.60 (m, 2H, CyHex), 1.59 (m, 5H, CH3 isop, CyHex), 1.41 (s, 3H, CH3 isop), 1.36 (m, 4H, CH3 isop, CyHex), 1.33 (s, 3H, CH3 isop), 1.27–1.15 (m, 3H, CyHex). Minor isomer: δ = 6.17 (d, J = 8.1 Hz, 1H, NH), 5.85 (d, J = 3.8 Hz, 1H, H-1 GlcF), 5.33 (d, J = 3.8 Hz, 1H, H-2 GlcF), 4.10–4.06 (m, 1H, H-6a GlcF), 4.02 (d, J = 9.3 Hz, 1H, H-5 GlcF), 3.97–3.91 (m, 2H, H-4, H-6b GlcF), 3.83–3.77 (m, 1H, CH-1 CyHex), 2.11 (s, 3H, CH3 Ac), 1.85 (ddd, J = 27.8, 12.4, 3.5 Hz, 2H, CyHex), 1.69–1.54 (m, 4H, CyHex), 1.50 (s, 3H, CH3 isop), 1.46 (s, 3H, CH3 isop), 1.41–1.33 (m, 1H, CyHex), 1.33 (s, 3H, CH3 isop), 1.31 (s, 3H, CH3 isop), 1.22–1.08 (m, 3H, CyHex). 13C NMR (CDCl3) Major isomer: δ = (170.0, 163.9) (CO), (114.0, 108.7) (C isop), 105.4 (C-1 GlcF), 85.6 (C-3 GlcF), 82.8 (C-2 GlcF), 82.3 (C-4 GlcF), 73.5 (C-5 GlcF), 65.0 (C-6 GlcF), 48.4 (CH CyHex), (32.7, 32.7) (CyHex), (27.0, 26.7, 26.5) (CH3 isop), 25.8 (CyHex), 25.5 (CH3 isop), (24.6, 24.5) (CyHex), 21.5(CH3 Ac). Minor isomer: δ = (169.0, 166.6) (CO), (112.8, 110.1) (C isop), 105.7 (C-1 GlcF), 85.3 (C-3 GlcF), 80.9 (C-2 GlcF), 80.4 (C-4 GlcF), 74.6 (C-5 GlcF), 68.4 (C-6 GlcF), 48.2 (CH CyHex), (33.1, 32.7) (CyHex), (27.1, 27.0, 26.8, 25.8) (CH3 isop), (25.7, 24.7, 24.7) (CyHex), 21.0 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C21H33NO8 [M + H]+ 428.2284; found 428.2302.
3.1.25. (3aR,4′R,7aR)-7-(Cyclohexylcarbamoyl)-2,2,2′,2′-tetramethyltetrahydrospiro[[1,3]dioxolo[4,5-c]pyran-6,4′-[1,3]dioxolan]-7-yl acetate (9a)
Yield 51% (22 mg); colorless oil; Rf (DS1) = 0.80, Rf (DS2) = 0.40 (EtOAc/petroleum ether 1:1, v/v); d.r. 55:45. 1H NMR (CDCl3) Major isomer: δ = 5.99 (d, J = 7.7 Hz, 1H, NH), 5.30 (d, J = 7.0 Hz, 1H, H-4 FruP), 4.60 (dt, J = 7.3, 3.7 Hz, 1H, H-5 FruP), 4.57 (d, J = 9.6 Hz, 1H, H-1a FruP), 4.07 (m, 1H, H-1b FruP), 4.05 (m, 1H, H-6a FruP), 3.80–3.74 (m, 1H, CH-1 CyHex), 3.68 (dd, J = 12.8, 3.4 Hz, 1H, H-6b FruP), 2.11 (s, 3H, CH3 Ac), 1.86 (t, J = 17.8 Hz, 2H, CyHex), 1.65–1.57 (m, 4H, CyHex), 1.47 (s, 6H, CH3 isop), 1.46 (s, 3H, CH3 isop), 1.38 (s, 1H, CyHex), 1.30 (s, 3H, CH3 isop), 1.21–1.06 (m, 3H, CyHex). Minor isomer: δ = 6.66 (d, J = 7.3 Hz, 1H, NH), 5.08 (d, J = 7.2 Hz, 1H, H-4 FruP), 4.31 (d, J = 9.9 Hz, 1H, H-1a FruP), 4.30–4.24 (m, 1H, H-5 FruP), 4.20 (dd, J = 12.6, 3.3 Hz, 1H, H-6a FruP), 3.93 (d, J = 9.9 Hz, 1H, H-1b FruP), 3.79 (dd, J = 12.6, 0.9 Hz, 1H, H-6b FruP), 3.77–3.73 (m, 1H, CyHex CH-1), 2.12 (s, 3H, CH3 Ac), 1.92 (dd, J = 33.6, 9.8 Hz, 2H, CyHex), 1.66 (m, 2H, CyHex), 1.58 (m, 2H, CyHex), 1.51 (s, 3H, CH3 isop), 1.49 (s, 3H, CH3 isop), 1.43 (s, 3H, CH3 isop), 1.36 (s, 3H, CH3 isop), 1.33 (m, 1H, CyHex), 1.23–1.12 (m, 3H, CyHex). 13C NMR (CDCl3) Major isomer: δ = 168.8, 166.6 (CO), 110.6, 109.5 (C isop), 104.5 (C-2 FruP), 80.5 (C-3 FruP), 73.1 (C-4 FruP), 72.8 (C-5 FruP), 72.3 (C-1 FruP), 64.7 (C-6 FruP), 48.3 (CH-1 CyHex), 32.9, 32.7 (CyHex), 26.9, 26.6, 26.1, 25.8, 25.0, 24.7, 24.6 (CH3 isop), 21.6 (CH3 Ac). Minor isomer: δ = 169.3, 164.6 (CO), 111.6, 110.8 (C isop), 103.9 (C-2 FruP), 81.0 (C-3 FruP), 73.60 (C-1 FruP), 73.4 (C-4 FruP), 73.0 (C-5 FruP), 64.4 (C-6 FruP), 48.9 (CH-1 CyHex), 32.9, 32.7 (CyHex), 26.4, 26.3, 26.2 (CH3 isop), 25.8 (CyHex), 25.3 (CH3 isop), 24.9 (CyHex), 21.7 (CH3 Ac). HRMS (MALDI–TOF/TOF) m/z: calcd. for C21H33NO8Na [M + Na]+ 450.2104; found 450.2107.