Detection of Antigen-Specific T Cells Using In Situ MHC Tetramer Staining
Abstract
:1. Introduction
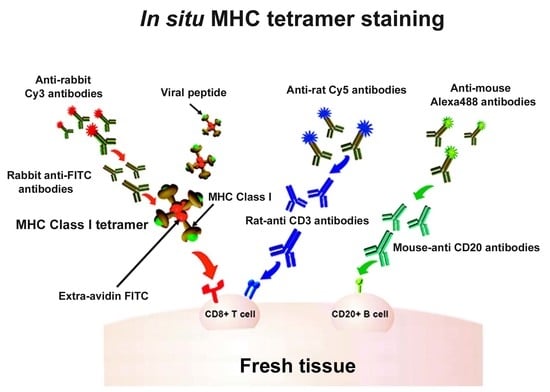
2. In Situ Tetramer Staining
3. Direct vs. Indirect IST
4. IST Staining on Fresh and Frozen Tissue
5. Specificity and Sensitivity of IST
6. Applications for In Situ Tetramer Staining
7. Limitations of IST Staining
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantin, C.M.; Bonney, E.E.; Altman, J.D.; Strickland, O.L. Major Histocompatibility Complex (MHC) Tetramer Technology: An Evaluation. Biol. Res. Nurs. 2002, 4, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.D.; Moss, P.A.H.; Goulder, P.J.R.; Barouch, D.H.; McHeyzer-Williams, M.G.; Bell, J.I.; McMichael, A.J.; Davis, M.M. Phenotypic Analysis of Antigen-Specific T Lymphocytes. Science 1996, 274, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Novak, E.J.; Liu, A.W.; Nepom, G.T.; Kwok, W.W. MHC Class II Tetramers Identify Peptide-Specific Human CD4+ T Cells Proliferating in Response to Influenza A Antigen. J. Clin. Investig. 1999, 104, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Goulder, P.J.R.; Watkins, D.I. Impact of MHC Class I Diversity on Immune Control of Immunodeficiency Virus Replication. Nat. Rev. Immunol. 2008, 8, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.J.; Schmitz, J.E.; Charini, W.A.; Nickerson, C.E.; Lifton, M.A.; Lord, C.I.; Forman, M.A.; Letvin, N.L. Emergence of CTL Coincides with Clearance of Virus during Primary Simian Immunodeficiency Virus Infection in Rhesus Monkeys. J. Immunol. 1999, 162, 5127–5133. [Google Scholar]
- Tjernlund, A.; Zhu, J.; Laing, K.; Diem, K.; McDonald, D.; Vazquez, J.; Cao, J.; Ohlen, C.; McElrath, M.J.; Picker, L.J.; et al. In Situ Detection of Gag-Specific CD8+cells in the GI Tract of SIV Infected Rhesus Macaques. Retrovirology 2010, 7, 15–17. [Google Scholar] [CrossRef]
- Loffredo, J.; Valentine, L.; Watkins, D. Beyond Mamu-A* 01+ Indian Rhesus Macaques: Continued Discovery of New MHC Class I Molecules That Bind Epitopes from the Simian AIDS Viruses. HIV Mol. Immunol. 2006, 29–51. [Google Scholar]
- Steinert, E.M.; Schenkel, J.M.; Fraser, K.A.; Beura, L.K.; Manlove, L.S.; Igyártó, B.Z.; Southern, P.J.; Masopust, D. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance HHS Public Access. Cell 2015, 161, 737–749. [Google Scholar] [CrossRef]
- Skinner, P.J.; Daniels, M.A.; Schmidt, C.S.; Jameson, S.C.; Haase, A.T. Cutting Edge: In Situ Tetramer Staining of Antigen-Specific T Cells in Tissues. J. Immunol. 2000, 165, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.B.; van Oijen, M.G.; Tirion, F.; Oomen, L.C.; Kruisbeek, A.M.; Vyth-Dreese, F.A.; Schumacher, T.N. In Situ Detection of Virus- and Tumor-Specific T-Cell Immunity. Nat. Med. 2000, 6, 1056–1060. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Zhou, L.; Fang, Y.; Huang, L.; Ren, L.; Peng, Y.; Li, Y.; Yang, F.; Xie, D.; et al. Use of HLA-DR*08032/E7 and HLA-DR*0818/E7 Tetramers in Tracking of Epitope-Specific CD4 + T Cells in Active and Convalescent Tuberculosis Patients Compared with Control Donors. Immunobiology 2011, 216, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Bischof, F.; Hofmann, M.; Schumacher, T.N.M.; Vyth-Dreese, F.A.; Weissert, R.; Schild, H.; Kruisbeek, A.M.; Melms, A. Analysis of Autoreactive CD4 T Cells in Experimental Autoimmune Encephalomyelitis after Primary and Secondary Challenge Using MHC Class II Tetramers. J. Immunol. 2004, 172, 2878–2884. [Google Scholar] [CrossRef] [PubMed]
- Dileepan, T.; Kim, H.O.; Cleary, P.P.; Skinner, P.J. In Situ Peptide-MHC-II Tetramer Staining of Antigen-Specific CD4+ T Cells in Tissues. PLoS ONE 2015, 10, e0128862. [Google Scholar] [CrossRef] [PubMed]
- Massilamany, C.; Gangaplara, A.; Jia, T.; Elowsky, C.; Li, Q.; Zhou, Y.; Reddy, J. In Situ Detection of Autoreactive CD4 T Cells in Brain and Heart Using Major Histocompatibility Complex Class II Dextramers. J. Vis. Exp. 2014, 90, 1–7. [Google Scholar] [CrossRef]
- Massilamany, C.; Gangaplara, A.; Jia, T.; Elowsky, C.; Kang, G.; Riethoven, J.J.; Li, Q.; Zhou, Y.; Reddy, J. Direct Staining with Major Histocompatibility Complex Class II Dextramers Permits Detection of Antigen-Specific, Autoreactive CD4 T Cells in Situ. PLoS ONE 2014, 9, e87519. [Google Scholar] [CrossRef]
- Li, S.; Mwakalundwa, G.; Skinner, P.J. In Situ MHC-Tetramer Staining and Quantitative Analysis to Determine the Location, Abundance, and Phenotype of Antigen-Specific CD8 T Cells in Tissues. J. Vis. Exp. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Skinner, P.J.; Haase, A.T. Skinner In Situ Tetramer Staining. J. Immunol. Methods 2002, 268, 29–34. [Google Scholar] [CrossRef]
- Benechet, A.P.; Menon, M.; Khanna, K.M. Visualizing T Cell Migration in Situ. Front. Immunol. 2014, 5, 363. [Google Scholar] [CrossRef]
- Skinner, P.J. In Situ MHC Tetramer Staining: In Situ Tetramers. In Analyzing T Cell Responses: How to Analyze Cellular Immune Responses against Tumor Associated Antigens; Springer: Amsterdam, The Netherlands, 2005; pp. 219–225. [Google Scholar] [CrossRef]
- Skinner, P.J.; Haase, A.T. In Situ Staining Using MHC Class I Tetramers. Curr. Protoc. Immunol. 2004, 64, 17. [Google Scholar] [CrossRef]
- Vyth-Dreese, F.A.; Kim, Y.H.; Dellemijn, T.A.M.; Schrama, E.; Haanen, J.B.A.G.; Spierings, E.; Goulmy, E. In Situ Visualization of Antigen-Specific T Cells in Cryopreserved Human Tissues. J. Immunol. Methods 2006, 310, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.; Pedersen, L.O.; Capeller, B.; Bröcker, E.B.; Becker, J.C.; thor Straten, P. Spontaneous Cytotoxic T-Cell Responses against Survivin-Derived MHC Class I-Restricted T-Cell Epitopes in Situ as Well as Ex Vivo in Cancer Patients. Cancer Res. 2001, 61, 5964–5968. [Google Scholar] [PubMed]
- Tjernlund, A.; Burgener, A.; Lindvall, J.M.; Peng, T.; Zhu, J.; Öhrmalm, L.; Picker, L.J.; Broliden, K.; Mcelrath, M.J.; Corey, L. In Situ Staining and Laser Capture Microdissection of Lymph Node Residing SIV Gag-Specific CD8 + T Cells-A Tool to Interrogate a Functional Immune Response Ex Vivo. PLoS ONE 2016, 11, e0149907. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, H.M.; Kim, H.O.; Wagstaff, R.; Sawahata, R.; Southern, P.J.; Skinner, P.J. Comparison of Vibratome and Compresstome Sectioning of Fresh Primate Lymphoid and Genital Tissues for in Situ MHC-Tetramer and Immunofluorescence Staining. Biol. Proced. Online 2015, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Skinner, P.J.; Ha, S.-J.; Duan, L.; Mattila, T.L.; Hage, A.; White, C.; Barber, D.L.; O’Mara, L.; Southern, P.J.; et al. Visualizing Antigen-Specific and Infected Cells in Situ Predicts Outcomes in Early Viral Infection. Science 2009, 323, 1726–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiniry, B.E.; Li, S.; Ganesh, A.; Hunt, P.W.; Somsouk, M.; Skinner, P.J.; Deeks, S.G.; Shacklett, B.L. Detection of HIV-1-Specific Gastrointestinal Tissue Resident CD8 + T-Cells in Chronic Infection. Mucosal Immunol. 2018, 11, 909–920. [Google Scholar] [CrossRef]
- Webb, G.M.; Li, S.; Mwakalundwa, G.; Folkvord, J.M.; Greene, J.M.; Reed, J.S.; Stanton, J.J.; Legasse, A.W.; Hobbs, T.; Martin, L.D.; et al. The Human IL-15 Superagonist ALT-803 Directs SIV-Specific CD8+ T Cells into B-Cell Follicles. Blood Adv. 2018, 2, 76–84. [Google Scholar] [CrossRef]
- Mylvaganam, G.H.; Rios, D.; Abdelaal, H.M.; Iyer, S.; Tharp, G.; Mavinger, M.; Hicks, S.; Chahroudi, A.; Ahmed, R.; Bosinger, S.E.; et al. Dynamics of SIV-Specific CXCR5+ CD8 T Cells during Chronic SIV Infection. Proc. Natl. Acad. Sci. USA 2017, 114, 1976–1981. [Google Scholar] [CrossRef]
- Reynolds, M.R.; Rakasz, E.; Skinner, P.J.; White, C.; Abel, K.; Ma, Z.-M.; Compton, L.; Napoe, G.; Wilson, N.; Miller, C.J.; et al. CD8+ T-Lymphocyte Response to Major Immunodominant Epitopes after Vaginal Exposure to Simian Immunodeficiency Virus: Too Late and Too Little. J. Virol. 2005, 79, 9228–9235. [Google Scholar] [CrossRef]
- Li, S.; Folkvord, J.M.; Rakasz, E.G.; Abdelaal, H.M.; Wagstaff, R.K.; Kovacs, K.J.; Kim, H.O.; Sawahata, R.; MaWhinney, S.; Masopust, D.; et al. Simian Immunodeficiency Virus-Producing Cells in Follicles Are Partially Suppressed by CD8 + Cells In Vivo. J. Virol. 2016, 90, 11168–11180. [Google Scholar] [CrossRef]
- Hong, J.J.; Reynolds, M.R.; Mattila, T.L.; Hage, A.; Watkins, D.I.; Miller, C.J.; Skinner, P.J. Localized Populations of CD8low/-MHC Class I Tetramer+ SIV-Specific T Cells in Lymphoid Follicles and Genital Epithelium. PLoS ONE 2009, 4, e4131. [Google Scholar] [CrossRef] [PubMed]
- Connick, E.; Folkvord, J.M.; Lind, K.T.; Rakasz, E.G.; Miles, B.; Wilson, N.A.; Santiago, M.L.; Schmitt, K.; Stephens, E.B.; Kim, H.O.; et al. Compartmentalization of Simian Immunodeficiency Virus Replication within Secondary Lymphoid Tissues of Rhesus Macaques Is Linked to Disease Stage and Inversely Related to Localization of Virus-Specific CTL. J. Immunol. 2014, 193, 5613–5625. [Google Scholar] [CrossRef] [PubMed]
- Connick, E.; Mattila, T.; Folkvord, J.M.; Schlichtemeier, R.; Meditz, A.L.; Ray, M.G.; McCarter, M.D.; MaWhinney, S.; Hage, A.; White, C.; et al. CTL Fail to Accumulate at Sites of HIV-1 Replication in Lymphoid Tissue. J. Immunol. 2007, 178, 6975–6983. [Google Scholar] [CrossRef] [PubMed]
- Sasikala-Appukuttan, A.K.; Kim, H.O.; Kinzel, N.J.; Hong, J.J.; Smith, A.J.; Wagstaff, R.; Reilly, C.; Piatak, M.; Lifson, J.D.; Reeves, R.K.; et al. Location and Dynamics of the Immunodominant CD8 T Cell Response to SIVΔnef Immunization and SIVmac251 Vaginal Challenge. PLoS ONE 2013, 8, e81623. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Folkvord, J.M.; Kovacs, K.J.; Wagstaff, R.K.; Mwakalundwa, G.; Rendahl, A.K.; Rakasz, E.G.; Connick, E.; Skinner, P.J. Low Levels of Siv-Specific CD8+ T Cells in Germinal Centers Characterizes Acute SIV Infection. PLoS Pathog. 2019, 15, e1007311. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, T.; Martin-Orozco, N.; Mallet-Designe, V.; Poirot, L.; McGavern, D.; Losyev, G.; Dobbs, C.M.; Oldstone, M.B.A.; Yoshida, K.; Kikutani, H.; et al. Susceptible MHC Alleles, Not Background Genes, Select an Autoimmune T Cell Reactivity. J. Clin. Investig. 2003, 112, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-X.; Wheatley, A.K.; Esterbauer, R.; Jegaskanda, S.; Glass, J.J.; Masopust, D.; De Rose, R.; Kent, S.J. Induction of Vaginal-Resident HIV-Specific CD8 T Cells with Mucosal Prime-Boost Immunization. Mucosal Immunol. 2017, 13, 994. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Y.; Fang, Y.; Wang, J.; Li, Y.; Wang, N.; Zhang, J.; Gao, M.; Huang, L.; Yang, F.; et al. Relatively Low Level of Antigen-Specific Monocytes Detected in Blood from Untreated Tuberculosis Patients Using CD4+ T-Cell Receptor Tetramers. PLoS Pathog. 2012, 8, e1001036. [Google Scholar] [CrossRef]
- Oerke, S.; Höhn, H.; Zehbe, I.; Pilch, H.; Schicketanz, K.H.; Hitzler, W.E.; Neukirch, C.; Freitag, K.; Maeurer, M.J. Naturally Processed and HLA-B8-Presented HPV16 E7 Epitope Recognized by T Cells from Patients with Cervical Cancer. Int. J. Cancer 2005, 114, 766–778. [Google Scholar] [CrossRef]
- Tully, G.; Kortsik, C.; Höhn, H.; Zehbe, I.; Hitzler, W.E.; Neukirch, C.; Freitag, K.; Kayser, K.; Maeurer, M.J. Highly Focused T Cell Responses in Latent Human Pulmonary Mycobacterium Tuberculosis Infection. J. Immunol. 2005, 174, 2174–2184. [Google Scholar] [CrossRef]
- De Vries, I.J.M.; Bernsen, M.R.; Van Geloof, W.L.; Scharenborg, N.M.; Lesterhuis, W.J.; Rombout, P.D.M.; Van Muijen, G.N.P.; Figdor, C.G.; Punt, C.J.A.; Ruiter, D.J.; et al. In Situ Detection of Antigen-Specific T Cells in Cryo-Sections Using MHC Class I Tetramers after Dendritic Cell Vaccination of Melanoma Patients. Cancer Immunol. Immunother. 2007, 56, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jaramillo, A.; Liu, W.; Olack, B.; Yoshimura, Y.; Joyce, S.; Kaleem, Z.; Mohanakumar, T. Chronic Rejection of Murine Cardiac Allografts Discordant at the H13 Minor Histocompatibility Antigen Correlates with the Generation of the H13-Specific CD8+ Cytotoxic T Cells. Transplantation 2003, 76, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Wang, H.; Starrett, G.J.; Phuong, V.; Jameson, S.C.; Hogquist, K.A. Tissue-Specific Distribution of INKT Cells Impacts Their Cytokine Response. Immunity 2015, 43, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, Y.; Lum, R.; Okoye, A.A.; Park, H.; Matsuda, K.; Bae, J.Y.; Hagen, S.I.; Shoemaker, R.; Deleage, C.; Lucero, C.; et al. B Cell Follicle Sanctuary Permits Persistent Productive Simian Immunodeficiency Virus Infection in Elite Controllers. Nat. Med. 2015, 21, 132–139. [Google Scholar] [CrossRef]
- Gratton, S.; Cheynier, R.; Dumaurier, M.J.; Oksenhendler, E.; Wain-Hobson, S. Highly Restricted Spread of HIV-1 and Multiply Infected Cells within Splenic Germinal Centers. Proc. Natl. Acad. Sci. USA 2000, 97, 14566–14571. [Google Scholar] [CrossRef]
- Perreau, M.; Savoye, A.-L.; De Crignis, E.; Corpataux, J.-M.; Cubas, R.; Haddad, E.K.; De Leval, L.; Graziosi, C.; Pantaleo, G. Follicular Helper T Cells Serve as the Major CD4 T Cell Compartment for HIV-1 Infection, Replication, and Production. J. Exp. Med. 2013, 210, 143–156. [Google Scholar] [CrossRef]
- Embretson, J.; Zupancic, M.; Ribas, J.L.; Burke, A.; Racz, P.; Tenner-Racz, K.; Haase, A.T. Massive Covert Infection of Helper T Lymphocytes and Macrophages by HIV during the Incubation Period of AIDS. Nature 1993, 362, 359–362. [Google Scholar] [CrossRef]
- Hufert, F.T.; van Lunzen, J.; Janossy, G.; Bertram, S.; Schmitz, J.; Haller, O.; Racz, P.; von Laer, D. Germinal Centre CD4+ T Cells Are an Important Site of HIV Replication in Vivo. AIDS 1997, 11, 849–857. [Google Scholar] [CrossRef]
- Folkvord, J.M.; Armon, C.; Connick, E. Lymphoid Follicles Are Sites of Heightened Human Immunodeficiency Virus Type 1 (HIV-1) Replication and Reduced Antiretroviral Effector Mechanisms. AIDS Res. Hum. Retrovir. 2005, 21, 363–370. [Google Scholar] [CrossRef]
- Haran, K.P.; Hajduczki, A.; Pampusch, M.S.; Mwakalundwa, G.; Vargas-Inchaustegui, D.A.; Rakasz, E.G.; Connick, E.; Berger, E.A.; Skinner, P.J. Simian Immunodeficiency Virus (SIV)-Specific Chimeric Antigen Receptor-T Cells Engineered to Target B Cell Follicles and Suppress SIV Replication. Front. Immunol. 2018, 9, 492. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Skinner, P.J.; Duan, L.; Haase, A.T. A Technique to Simultaneously Visualize Virus-Specific CD8+ T Cells and Virus-Infected Cells in Situ. J. Vis. Exp. 2009, 30, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Kumamoto, Y.; Gopinath, S.; Iwasaki, A. CD301b+ Dendritic Cells Stimulate Tissue-Resident Memory CD8+ T Cells to Protect against Genital HSV-2. Nat. Commun. 2016, 7, 13346. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.M.; McNamara, J.T.; Lefrançois, L. In Situ Imaging of the Endogenous CD8 T Cell Response to Infection. Science 2007, 318, 116–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppieters, K.T.; Dotta, F.; Amirian, N.; Campbell, P.D.; Kay, T.W.H.; Atkinson, M.A.; Roep, B.O.; von Herrath, M.G. Demonstration of Islet-Autoreactive CD8 T Cells in Insulitic Lesions from Recent Onset and Long-Term Type 1 Diabetes Patients. J. Exp. Med. 2012, 209, 51–60. [Google Scholar] [CrossRef]
- Kaslow, R.A.; Carrington, M.; Apple, R.; Park, L.; Muñoz, A.; Saah, A.J.; Goedert, J.J.; Winkler, C.; O’Brien, S.J.; Rinaldo, C.; et al. Influence of Combinations of Human Major Histocompatibility Complex Genes on the Course of HIV-1 Infection. Nat. Med. 1996, 2, 405–411. [Google Scholar] [CrossRef]
- Migueles, S.A.; Sabbaghian, M.S.; Shupert, W.L.; Bettinotti, M.P.; Marincola, F.M.; Martino, L.; Hallahan, C.W.; Selig, S.M.; Schwartz, D.; Sullivan, J.; et al. HLA B*5701 Is Highly Associated with Restriction of Virus Replication in a Subgroup of HIV-Infected Long Term Nonprogressors. Proc. Natl. Acad. Sci. USA 2000, 97, 2709–2714. [Google Scholar] [CrossRef]
- Mothe, B.R.; Weinfurter, J.; Wang, C.; Rehrauer, W.; Wilson, N.; Allen, T.M.; Allison, D.B.; Watkins, D.I. Expression of the Major Histocompatibility Complex Class I Molecule Mamu-A*01 Is Associated with Control of Simian Immunodeficiency Virus SIVmac239 Replication. J. Virol. 2003, 77, 2736–2740. [Google Scholar] [CrossRef]
- Loffredo, J.T.; Maxwell, J.; Qi, Y.; Glidden, C.E.; Borchardt, G.J.; Soma, T.; Bean, A.T.; Beal, D.R.; Wilson, N.A.; Rehrauer, W.M.; et al. Mamu-B*08-Positive Macaques Control Simian Immunodeficiency Virus Replication. J. Virol. 2007, 81, 8827–8832. [Google Scholar] [CrossRef]
- Yant, L.J.; Friedrich, T.C.; Johnson, R.C.; May, G.E.; Maness, N.J.; Enz, A.M.; Lifson, J.D.; O’Connor, D.H.; Carrington, M.; Watkins, D.I. The High-Frequency Major Histocompatibility Complex Class I Allele Mamu-B*17 Is Associated with Control of Simian Immunodeficiency Virus SIVmac239 Replication. J. Virol. 2006, 80, 5074–5077. [Google Scholar] [CrossRef]
- Laugel, B.; Van Den Berg, H.A.; Gostick, E.; Cole, D.K.; Wooldridge, L.; Boulter, J.; Milicic, A.; Price, D.A.; Sewell, A.K. Different T Cell Receptor Affinity Thresholds and CD8 Coreceptor Dependence Govern Cytotoxic T Lymphocyte Activation and Tetramer Binding Properties. J. Biol. Chem. 2007, 282, 23799–23810. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.J.; Andargachew, R.; Martinez, H.A.; Evavold, B.D. Low-Affinity CD4+ T Cells Are Major Responders in the Primary Immune Response. Nat. Commun. 2016, 7, 13848. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.J.; Evavold, B.D. Lower Affinity T Cells Are Critical Components and Active Participants of the Immune Response. Front. Immunol. 2015, 6, 468. [Google Scholar] [CrossRef] [PubMed]
- Rius, C.; Attaf, M.; Tungatt, K.; Bianchi, V.; Legut, M.; Bovay, A.; Donia, M.; thor Straten, P.; Peakman, M.; Svane, I.M.; et al. Peptide–MHC Class I Tetramers Can Fail to Detect Relevant Functional T Cell Clonotypes and Underestimate Antigen-Reactive T Cell Populations. J. Immunol. 2018, 200, 2263–2279. [Google Scholar] [CrossRef] [PubMed]
- Dolton, G.; Lissina, A.; Skowera, A.; Ladell, K.; Tungatt, K.; Jones, E.; Kronenberg-Versteeg, D.; Akpovwa, H.; Pentier, J.M.; Holland, C.J.; et al. Comparison of Peptide-Major Histocompatibility Complex Tetramers and Dextramers for the Identification of Antigen-Specific T Cells. Clin. Exp. Immunol. 2014, 177, 47–63. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaal, H.M.; Cartwright, E.K.; Skinner, P.J. Detection of Antigen-Specific T Cells Using In Situ MHC Tetramer Staining. Int. J. Mol. Sci. 2019, 20, 5165. https://doi.org/10.3390/ijms20205165
Abdelaal HM, Cartwright EK, Skinner PJ. Detection of Antigen-Specific T Cells Using In Situ MHC Tetramer Staining. International Journal of Molecular Sciences. 2019; 20(20):5165. https://doi.org/10.3390/ijms20205165
Chicago/Turabian StyleAbdelaal, Hadia M., Emily K. Cartwright, and Pamela J. Skinner. 2019. "Detection of Antigen-Specific T Cells Using In Situ MHC Tetramer Staining" International Journal of Molecular Sciences 20, no. 20: 5165. https://doi.org/10.3390/ijms20205165