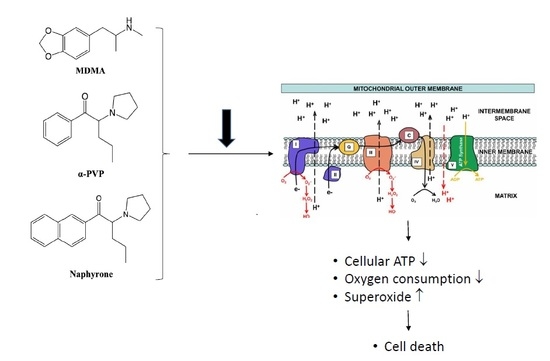
Molecular Toxicological Mechanisms of Synthetic Cathinones on C2C12 Myoblasts
Abstract
:1. Introduction
2. Results
2.1. Cell Viability and ATP Content
2.2. Effect on Cellular Oxygen Consumption
2.3. Activity of Enzyme Complexes of the Mitochondrial Electron Transport Chain
2.4. Mitochondrial Superoxide Production
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Plasma Membrane Integrity
4.4. ATP Content
4.5. Oxygen Consumption
4.6. Activity of Specific Enzyme Complexes of the Mitochondrial Electron Transport Chain
4.7. Mitochondrial Superoxide Production
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2017. Available online: http://www.emcdda.europa.eu/system/files/publications/4541/TDAT17001ENN.pdf (accessed on 4 October 2018).
- Prosser, J.M.; Nelson, L.S. The toxicology of bath salts: A review of synthetic cathinones. J. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S. Sur un homologue de l’ephedrine. Bull. Soc. Chim. France 1929, 45, 284–286. [Google Scholar]
- Valente, M.J.; Guedes de Pinho, P.; de Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Soroko, F.E.; Mehta, N.B.; Maxwell, R.A.; Ferris, R.M.; Schroeder, D.H. Bupropion hydrochloride ((+/-) alpha-t-butylamino-3-chloropropiophenone HCl): A novel antidepressant agent. J. Pharm. Pharmacol. 1977, 29, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Seaton, D.A.; Duncan, L.J.; Rose, K.; Scott, A.M. Diethyl-propion in the treatment of “refractory” obesity. Br. Med. J. 1961, 1, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, G. Diethylpropion in the treatment of obesity. J. Coll. Gen. Pract. 1963, 6, 347. [Google Scholar] [PubMed]
- Canning, H.; Goff, D.; Leach, M.J.; Miller, A.A.; Tateson, J.E.; Wheatley, P.L. The involvement of dopamine in the central actions of bupropion, a new antidepressant [proceedings]. Br. J. Pharmacol. 1979, 66, 104p–105p. [Google Scholar] [PubMed]
- Dal Cason, T.A.; Young, R.; Glennon, R.A. Cathinone: An investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol. Biochem. Behav. 1997, 58, 1109–1116. [Google Scholar] [CrossRef]
- Simmler, L.D.; Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 2014, 79, 152–160. [Google Scholar] [CrossRef]
- Simmler, L.D.; Buser, T.A.; Donzelli, M.; Schramm, Y.; Dieu, L.H.; Huwyler, J.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: Para-halogenated amphetamines and pyrovalerone cathinones. Eur. Neuropsychopharmacol. 2015, 25, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Mayer, F.P.; Wimmer, L.; Dillon-Carter, O.; Partilla, J.S.; Burchardt, N.V.; Mihovilovic, M.D.; Baumann, M.H.; Sitte, H.H. Phase I metabolites of mephedrone display biological activity as substrates at monoamine transporters. Br. J. Pharmacol. 2016, 173, 2657–2668. [Google Scholar] [CrossRef] [Green Version]
- Luethi, D.; Kolaczynska, K.E.; Docci, L.; Krahenbuhl, S.; Hoener, M.C.; Liechti, M.E. Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology 2018, 134, 4–12. [Google Scholar] [CrossRef]
- Baumann, M.H.; Ayestas, M.A., Jr.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef]
- Wood, D.M.; Davies, S.; Greene, S.L.; Button, J.; Holt, D.W.; Ramsey, J.; Dargan, P.I. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin. Toxicol. 2010, 48, 924–927. [Google Scholar] [CrossRef]
- James, D.; Adams, R.D.; Spears, R.; Cooper, G.; Lupton, D.J.; Thompson, J.P.; Thomas, S.H. Clinical characteristics of mephedrone toxicity reported to the U.K. National Poisons Information Service. Emerg. Med. J. 2011, 28, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Beck, O.; Franzen, L.; Backberg, M.; Signell, P.; Helander, A. Intoxications involving MDPV in Sweden during 2010-2014: Results from the STRIDA project. Clin. Toxicol. 2015, 53, 865–873. [Google Scholar] [CrossRef]
- Beck, O.; Franzen, L.; Backberg, M.; Signell, P.; Helander, A. Toxicity evaluation of alpha-pyrrolidinovalerophenone (α-PVP): Results from intoxication cases within the STRIDA project. Clin. Toxicol. 2016, 54, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Umebachi, R.; Aoki, H.; Sugita, M.; Taira, T.; Wakai, S.; Saito, T.; Inokuchi, S. Clinical characteristics of alpha-pyrrolidinovalerophenone (α-PVP) poisoning. Clin. Toxicol. 2016, 54, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, S.; Lambe, E.; O’Dea, J. Acute liver failure following recreational use of psychotropic “head shop” compounds. Ir. J. Med. Sci. 2011, 180, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Borek, H.A.; Holstege, C.P. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3, 4-methylenedioxypyrovalerone. Ann. Emerg. Med. 2012, 60, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Penders, T.M.; Gestring, R.E.; Vilensky, D.A. Excited delirium following use of synthetic cathinones (bath salts). Gen. Hosp. Psychiatry 2012, 34, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.A.; Watson, M.; Goldberger, B. “Bath salts” intoxication. N. Engl. J. Med. 2011, 365, 967–968. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.A.; Reisfield, G.M.; Watson, M.C.; Chronister, C.W.; Goldberger, B.A. Psychoactive “bath salts” intoxication with methylenedioxypyrovalerone. Am. J. Med. 2012, 125, 854–858. [Google Scholar] [CrossRef]
- Patel, N.B. Mechanism of action of cathinone: The active ingredient of khat (Catha Edulis). East Afr. Med. J. 2000, 77, 329–332. [Google Scholar] [CrossRef]
- White, C.M. Mephedrone and 3,4-methylenedioxypyrovalerone (MDPV): Synthetic cathinones with serious health implications. J. Clin. Pharmacol. 2016, 56, 1319–1325. [Google Scholar] [CrossRef]
- Valente, M.J.; Araujo, A.M.; Silva, R.; Bastos Mde, L.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. 3,4-Methylenedioxypyrovalerone (MDPV): In vitro mechanisms of hepatotoxicity under normothermic and hyperthermic conditions. Arch. Toxicol. 2016, 90, 1959–1973. [Google Scholar] [CrossRef]
- Valente, M.J.; Araujo, A.M.; Bastos Mde, L.; Fernandes, E.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. characterization of hepatotoxicity mechanisms triggered by designer cathinone drugs (beta-keto amphetamines). Toxicol. Sci. 2016, 153, 89–102. [Google Scholar] [CrossRef]
- Luethi, D.; Liechti, M.E.; Krahenbuhl, S. Mechanisms of hepatocellular toxicity associated with new psychoactive synthetic cathinones. Toxicology 2017, 387, 57–66. [Google Scholar] [CrossRef]
- Valente, M.J.; Bastos, M.L.; Fernandes, E.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. Neurotoxicity of beta-keto amphetamines: Deathly mechanisms elicited by methylone and MDPV in human dopaminergic SH-SY5Y cells. ACS Chem. Neurosci. 2017, 8, 850–859. [Google Scholar] [CrossRef]
- Valente, M.J.; Amaral, C.; Correia-da-Silva, G.; Duarte, J.A.; Bastos, M.L.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. Methylone and MDPV activate autophagy in human dopaminergic SH-SY5Y cells: A new insight into the context of beta-keto amphetamines-related neurotoxicity. Arch. Toxicol. 2017, 91, 3663–3676. [Google Scholar] [CrossRef] [PubMed]
- Halachanova, V.; Sansone, R.A.; McDonald, S. Delayed rhabdomyolysis after ecstasy use. Mayo Clin. Proc. 2001, 76, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.A.; Leao, A.; Magalhaes, J.; Ascensao, A.; Bastos, M.L.; Amado, F.L.; Vilarinho, L.; Quelhas, D.; Appell, H.J.; Carvalho, F. Strenuous exercise aggravates MDMA-induced skeletal muscle damage in mice. Toxicology 2005, 206, 349–358. [Google Scholar] [CrossRef]
- Carvalho, M.; Carmo, H.; Costa, V.M.; Capela, J.P.; Pontes, H.; Remiao, F.; Carvalho, F.; Bastos Mde, L. Toxicity of amphetamines: An update. Arch. Toxicol. 2012, 86, 1167–1231. [Google Scholar] [CrossRef]
- Vanden Eede, H.; Montenij, L.J.; Touw, D.J.; Norris, E.M. Rhabdomyolysis in MDMA intoxication: A rapid and underestimated killer. “Clean” ecstasy, a safe party drug? J. Emerg. Med. 2012, 42, 655–658. [Google Scholar] [CrossRef]
- Kelly, O.M.; McNamara, Y.M.; Manzke, L.H.; Meegan, M.J.; Porter, R.K. The preservation of in vivo phosphorylated and activated uncoupling protein 3 (UCP3) in isolated skeletal muscle mitochondria following administration of 3,4-methylenedioxymethamphetamine (MDMA aka ecstasy) to rats/mice. Mitochondrion 2012, 12, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felser, A.; Blum, K.; Lindinger, P.W.; Bouitbir, J.; Krähenbühl, S. Mechanisms of hepatocellular toxicity associated with dronedarone—A comparison to amiodarone. Toxicol. Sci. 2013, 131, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Paech, F.; Bouitbir, J.; Krahenbuhl, S. Hepatocellular Toxicity Associated with Tyrosine Kinase Inhibitors: Mitochondrial Damage and Inhibition of Glycolysis. Front. Pharmacol. 2017, 8, 367. [Google Scholar] [CrossRef]
- Flynn, J.M.; Melov, S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic. Biol. Med. 2013, 62, 4–12. [Google Scholar] [CrossRef]
- Torrance, H.; Cooper, G. The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland. Forensic Sci Int. 2010, 202, e62-3. [Google Scholar] [CrossRef]
- Kamalian, L.; Chadwick, A.E.; Bayliss, M.; French, N.S.; Monshouwer, M.; Snoeys, J.; Park, B.K. The utility of HepG2 cells to identify direct mitochondrial dysfunction in the absence of cell death. Toxicol. In Vitro 2015, 29, 732–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fromenty, B.; Fisch, C.; Berson, A.; Letteron, P.; Larrey, D.; Pessayre, D. Dual effect of amiodarone on mitochondrial respiration. Initial protonophoric uncoupling effect followed by inhibition of the respiratory chain at the levels of complex I and complex II. J. Pharmacol. Exp. Ther. 1990, 255, 1377–1384. [Google Scholar] [PubMed]
- Krähenbühl, S. Mitochondria: Important target for drug toxicity? J. Hepatol. 2001, 34, 334–336. [Google Scholar] [CrossRef]
- Serviddio, G.; Bellanti, F.; Giudetti, A.M.; Gnoni, G.V.; Capitanio, N.; Tamborra, R.; Romano, A.D.; Quinto, M.; Blonda, M.; Vendemiale, G.; et al. Mitochondrial oxidative stress and respiratory chain dysfunction account for liver toxicity during amiodarone but not dronedarone administration. Free Radic. Biol. Med. 2011, 51, 2234–2242. [Google Scholar] [CrossRef]
- Mills, E.M.; Banks, M.L.; Sprague, J.E.; Finkel, T. Pharmacology: Uncoupling the agony from ecstasy. Nature 2003, 426, 403–404. [Google Scholar] [CrossRef]
- Mills, E.M.; Rusyniak, D.E.; Sprague, J.E. The role of the sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4-methylenedioxymethamphetamine. J. Mol. Med. 2004, 82, 787–799. [Google Scholar] [CrossRef]
- Shortall, S.E.; Green, A.R.; Swift, K.M.; Fone, K.C.; King, M.V. Differential effects of cathinone compounds and MDMA on body temperature in the rat, and pharmacological characterization of mephedrone-induced hypothermia. Br. J. Pharmacol. 2013, 168, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Votyakova, T.V.; Reynolds, I.J. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001, 79, 266–277. [Google Scholar] [CrossRef]
- Drose, S.; Brandt, U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 2012, 748, 145–169. [Google Scholar]
- Antico Arciuch, V.G.; Elguero, M.E.; Poderoso, J.J.; Carreras, M.C. Mitochondrial regulation of cell cycle and proliferation. Antioxid. Redox Signal. 2012, 16, 1150–1180. [Google Scholar] [CrossRef]
- Meltzer, P.C.; Butler, D.; Deschamps, J.R.; Madras, B.K. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: A promising class of monoamine uptake inhibitors. J. Med. Chem. 2006, 49, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Sanvee, G.M.; Brecht, K.; Kratschmar, D.V.; Odermatt, A.; Bouitbir, J.; Krähenbühl, S. IGF-1 prevents simvastatin-induced myotoxicity in C2C12 myotubes. Arch. Toxicol. 2017, 91, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar] [PubMed]





| MDMA | 3-MMC | Mephedrone | Methylone | α-PVP | MDPV | Naphyrone | |
|---|---|---|---|---|---|---|---|
| Membrane toxicity | 0.74 | >2 | >2 | 1.10 | 1.04 | >2 | 0.047 |
| ATP depletion | 0.88 | 1.08 | 0.53 | 1.16 | 0.94 | 1.01 | 0.021 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Luethi, D.; Sanvee, G.M.; Bouitbir, J.; Liechti, M.E.; Krähenbühl, S. Molecular Toxicological Mechanisms of Synthetic Cathinones on C2C12 Myoblasts. Int. J. Mol. Sci. 2019, 20, 1561. https://doi.org/10.3390/ijms20071561
Zhou X, Luethi D, Sanvee GM, Bouitbir J, Liechti ME, Krähenbühl S. Molecular Toxicological Mechanisms of Synthetic Cathinones on C2C12 Myoblasts. International Journal of Molecular Sciences. 2019; 20(7):1561. https://doi.org/10.3390/ijms20071561
Chicago/Turabian StyleZhou, Xun, Dino Luethi, Gerda M. Sanvee, Jamal Bouitbir, Matthias E. Liechti, and Stephan Krähenbühl. 2019. "Molecular Toxicological Mechanisms of Synthetic Cathinones on C2C12 Myoblasts" International Journal of Molecular Sciences 20, no. 7: 1561. https://doi.org/10.3390/ijms20071561