Clinical and Biological Characterization of Skin Pigmentation Diversity and Its Consequences on UV Impact
Abstract
:1. Skin Pigmentation Diversity
1.1. Evolution of Skin Pigmentation Diversity
1.2. Melanocyte Biology
1.3. Genetic Basis of Skin Pigmentation Diversity
1.4. Clinical Classification of Skin Pigmentation
1.4.1. Fitzpatrick Classification
1.4.2. Individual Typology Angle-Based Skin Color Type Classification
1.4.3. Specialized Narrow-Band Reflectometry
1.4.4. Diffuse Reflectance Spectroscopy
1.5. Analysis of Melanins and Melanosomes in Skins of Variable Pigmentation
1.5.1. Melanin Content
1.5.2. Melanin Type
1.5.3. Melanosome Transfer, Distribution and Organization in Skins of Variable Pigmentation
1.6. Other Biological Factors that Influence Skin Constitutive Pigmentation
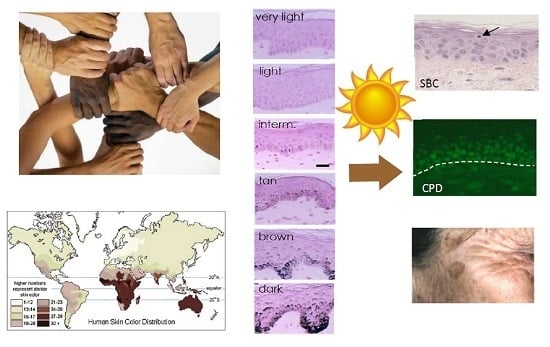
2. Acute Consequences of UV Exposure
2.1. Erythemal Reaction/Sunburn
2.2. DNA Damage
2.3. Pigmentation Induced by Sun Exposure
3. Mid- and Long-Term Consequences of UV Exposure
3.1. Skin Cancer
3.2. Photoaging
3.3. Skin Pigmentary Disorders Linked to Sun Exposure
3.3.1. Melasma
3.3.2. Post-Inflammatory Hyperpigmentation (PIH)
3.3.3. Seborrheic Keratosis (SK)
3.3.4. Solar Lentigo
3.3.5. Management of Hyperpigmented Disorders Linked to Sun Light
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-SCD | 2-S-cysteinyldopa |
| 4-AHP | 4-Amino-3-hydroxyphenylalanine |
| 5-SCD | 5-S-cysteinyldopa |
| ACTH | Adrenocorticotropic hormone |
| ADAM17 | ADAM metallopeptidase domain 17 |
| ADAMTS20 | ADAM metallopeptidase with thrombospondin type 1 motif 20 |
| AKT1 | AKT Serine/Threonine Kinase 1 |
| ASIP | Agouti signaling protein |
| ATRN | Attractin |
| BCC | Basal cell carcinoma |
| BED | Biologically efficient dose |
| bFGF | Basic FGF |
| BNC2 | Basonuclin 2 |
| CIE | Commission Internationale de l’Eclairage |
| CPD | Cyclobutane pyrimidine dimer |
| DCT | DOPA chrome tautomerase |
| DDB1 | Damage specific DNA binding protein 1 |
| DHI | 5,6-Dihydroxyindole |
| DHICA | 5,6-Dihydroxyindole-2-carboxylic acid |
| DNA | Deoxyribonucleic acid |
| DNA-PF | DNA-Protection factor |
| DOPA | l-3,4-dihydroxyphenyilalanine |
| DRS | Diffuse reflectance spectroscopy |
| DT | Delayed tanning |
| DTNBP1 | Dystrobrevin binding protein 1 |
| ECM | Extracellular matrix |
| EDA | Ectodysplasin A |
| EDN3 | Endothelin 3 |
| EGFR | Epidermal growth factor receptor |
| EI | Erythema index |
| FGFR3 | Fibroblast growth factor receptor 3 |
| FOXN1 | Forkhead box N1 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GWAS | Genome-wide association study |
| HERC2 | HECT And RLD domain containing E3 ubiquitin protein ligase 2 |
| HGF | Hepatocyte growth factor |
| HLA-C | Major histocompatibility complex, class I, C |
| HPLC | High-Performance liquid chromatography |
| IL1α | Interleukin 1 alpha |
| IPD | Immediate pigment darkening |
| IR | Infrared radiation |
| IRF4 | Interferon regulatory Factor 4 |
| ITA | Individual typology angle |
| KITLG | KIT ligand |
| LYST | Lysosomal trafficking regulator |
| MATP | Solute carrier family 45, member 2 |
| MC1R | Melanocortin-1 receptor |
| MED | Minimal erythemal dose |
| MFSD12 | Major facilitator superfamily domain containing 12 |
| MI | Melanin index |
| MITF | Microphthalmia-associated transcription factor |
| MM | Malignant melanoma |
| MYO5A | Myosin VA |
| OCA1 | Oculocutaneous albinism type 1 |
| OCA2 | Oculocutaneous albinism type 2 |
| OPN3 | Opsin 3 |
| PIH | Postinflammatory hyperpigmentation |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| PKA | Protein kinase A |
| POMC | Proopiomelanocortin |
| PPD | Persistent pigment darkening |
| PTCA | Pyrrole-2,3,5-tricarboxylic acid |
| ROS | Reactive oxygen species |
| SBC | Sunburn cell |
| SCC | Squamous cell carcinoma |
| SK | Seborrheic Keratosis |
| SLC24A5 | Solute carrier family 24, member 5 |
| SLC45A2 | Solute carrier family 45, member 2 |
| SMARCA2 | SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily A, member 2 |
| SNP | Single nucleotide polymorphism |
| SNX13 | Sorting nexin 13 |
| SPF | Sun protection factor |
| SPT | Skin phototype |
| SSR | Solar simulated radiation |
| TERT | Telomerase reverse transcriptase |
| TMEM38 | Transmembrane protein 38 |
| TPCN2 | Two pore segment channel 2 |
| TRP1 | Tyrosinase-related protein 1 |
| TRP2 | Tyrosinase-related protein 2 |
| TTCA | Thiazole-2,4,5-tricarboxylic acid |
| TYR | Tyrosinase |
| UVA | Ultraviolet radiation A |
| UVB | Ultraviolet radiation B |
| UVR | Ultraviolet radiation |
| VLDLR | Very low density lipoprotein receptor |
| αMSH | Alpha-Melanocyte-stimulating hormone |
References
- Jablonski, N.G.; Chaplin, G. Colloquium paper: Human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. 2), 8962–8968. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A D-Lightful health perspective. Nutr. Rev. 2008, 66, S182–S194. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, B.; Beleza, S.; Shriver, M.D. The genetic architecture of normal variation in human pigmentation: An evolutionary perspective and model. Hum. Mol. Genet. 2006, 15, (Spec No 2). R176–R181. [Google Scholar] [CrossRef] [PubMed]
- Alaluf, S.; Heath, A.; Carter, N.; Atkins, D.; Mahalingam, H.; Barrett, K.; Kolb, R.; Smit, N. Variation in melanin content and composition in type V and VI photoexposed and photoprotected human skin: The dominant role of DHI. Pigment Cell Res. 2001, 14, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B.; Breathnach, A.S. The epidermal melanin unit system. Dermatol. Wochenschr. 1963, 147, 481–489. [Google Scholar] [PubMed]
- Whiteman, D.C.; Parsons, P.G.; Green, A.C. Determinants of melanocyte density in adult human skin. Arch. Dermatol. Res. 1999, 291, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Stierner, U.; Rosdahl, I.; Augustsson, A.; Kagedal, B. UVB irradiation induces melanocyte increase in both exposed and shielded human skin. J. Investig. Dermatol. 1989, 92, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Alaluf, S.; Barrett, K.; Blount, M.; Carter, N. Ethnic variation in tyrosinase and TYRP1 expression in photoexposed and photoprotected human skin. Pigment Cell Res. 2003, 16, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Alaluf, S.; Atkins, D.; Barrett, K.; Blount, M.; Carter, N.; Heath, A. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002, 15, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Barsh, G.S. What controls variation in human skin color? PLoS Biol. 2003, 1, e27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Montagna, W.; Carlisle, K. The architecture of black and white facial skin. J. Am. Acad. Dermatol. 1991, 24, 929–937. [Google Scholar] [CrossRef]
- Taylor, S.C. Skin of color: Biology, structure, function, and implications for dermatologic disease. J. Am. Acad. Dermatol. 2002, 46, S41–S62. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Human hair melanins: What we have learned and have not learned from mouse coat color pigmentation. Pigment Cell Melanoma Res. 2011, 24, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Ohtara, K.; Ito, S. Chemical analysis of late stages of pheomelanogenesis: Conversion of dihydrobenzothiazine to a benzothiazole structure. Pigment Cell Melanoma Res. 2009, 22, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Suzuki, N.; Takebayashi, S.; Commo, S.; Wakamatsu, K. Neutral pH and copper ions promote eumelanogenesis after the dopachrome stage. Pigment Cell Melanoma Res. 2013, 26, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.Z.; Hill, G.J. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000, 13 (Suppl. 8), 140–144. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.Z.; Li, W.; Xin, P.; Mitchell, D.L. Melanin: A two edged sword? Pigment Cell Res. 1997, 10, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Kadekaro, A.L.; Kavanagh, R.J.; Wakamatsu, K.; Ito, S.; Pipitone, M.A.; Abdel-Malek, Z.A. Cutaneous photobiology. The melanocyte vs. the sun: Who will win the final round? Pigment Cell Res. 2003, 16, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Panzella, L.; Monfrecola, G.; d’Ischia, M. Pheomelanin-induced oxidative stress: Bright and dark chemistry bridging red hair phenotype and melanoma. Pigment Cell Melanoma Res. 2014, 27, 721–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissy, R.; Huizing, M.; Gahl, W.A. Biogenesis of melanosomes. In The Pigmentary System, 2nd ed.; Nordlund, J., Boissy, R., Hearing, V., King, R.A., Oetting, W., Ortonne, J.P., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2006; p. 155. [Google Scholar]
- Fuller, B.B.; Spaulding, D.T.; Smith, D.R. Regulation of the catalytic activity of preexisting tyrosinase in black and Caucasian human melanocyte cell cultures. Exp. Cell Res. 2001, 262, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Spaulding, D.T.; Glenn, H.M.; Fuller, B.B. The relationship between Na(+)/H(+) exchanger expression and tyrosinase activity in human melanocytes. Exp Cell Res. 2004, 298, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Corn, T.; Iwata, S.; Everett, M.A.; Fuller, B.B. The relationship between tyrosinase activity and skin color in human foreskins. J. Investig. Dermatol. 1990, 95, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Escobar, I.E.; Oancea, E. A melanosomal two-pore sodium channel regulates pigmentation. Sci. Rep. 2016, 6, 26570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.L. The melanocortin 1 receptor (MC1R): More than just red hair. Pigment Cell Res. 2000, 13, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Sturm, R.A.; Duffy, D.L. Human pigmentation genes under environmental selection. Genome Biol. 2012, 13, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturm, R.A. Molecular genetics of human pigmentation diversity. Hum. Mol. Genet. 2009, 18, R9–R17. [Google Scholar] [CrossRef] [PubMed]
- Sulem, P.; Gudbjartsson, D.F.; Stacey, S.N.; Helgason, A.; Rafnar, T.; Magnusson, K.P.; Manolescu, A.; Karason, A.; Palsson, A.; Thorleifsson, G.; et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat. Genet. 2007, 39, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Norton, H.L.; Kittles, R.A.; Parra, E.; McKeigue, P.; Mao, X.; Cheng, K.; Canfield, V.A.; Bradley, D.G.; McEvoy, B.; Shriver, M.D. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol. Biol. Evol. 2007, 24, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Basu Mallick, C.; Iliescu, F.M.; Mols, M.; Hill, S.; Tamang, R.; Chaubey, G.; Goto, R.; Ho, S.Y.; Gallego Romero, I.; Crivellaro, F.; et al. The light skin allele of SLC24A5 in South Asians and Europeans shares identity by descent. PLoS Genet. 2013, 9, e1003912. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.G.; Kelly, D.E.; Hansen, M.E.B.; Beltrame, M.H.; Fan, S.; Bowman, S.L.; Jewett, E.; Ranciaro, A.; Thompson, S.; Lo, Y.; et al. Loci associated with skin pigmentation identified in African populations. Science 2017, 358. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Lin, M.; Granka, J.M.; Myrick, J.W.; Liu, X.; Sockell, A.; Atkinson, E.G.; Werely, C.J.; Möller, M.; Sandhu, M.S.; et al. An Unexpectedly Complex Architecture for Skin Pigmentation in Africans. Cell 2017, 171, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Bigham, A.; Tan, J.; Li, S.; Gozdzik, A.; Ross, K.; Jin, L.; Parra, E.J. Association of the OCA2 polymorphism His615Arg with melanin content in East Asian populations: Further evidence of convergent evolution of skin pigmentation. PLoS Genet. 2010, 6, e1000867. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhong, H.; Chen, J.; Zhang, X.; Zhang, H.; Luo, X.; Xu, S.; Chen, H.; Lu, D.; Han, Y.; et al. A Genetic Mechanism for Convergent Skin Lightening during Recent Human Evolution. Mol. Biol. Evol. 2016, 33, 1177–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilla, C.; Boxill, L.A.; Donald, S.A.; Williams, T.; Sylvester, N.; Parra, E.J.; Dios, S.; Norton, H.L.; Shriver, M.D.; Kittles, R.A. The 8818G allele of the agouti signaling protein (ASIP) gene is ancestral and is associated with darker skin color in African Americans. Hum. Genet. 2005, 116, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kraft, P.; Nan, H.; Guo, Q.; Chen, C.; Qureshi, A.; Hankinson, S.E.; Hu, F.B.; Duffy, D.L.; Zhao, Z.Z.; et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008, 4, e1000074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valverde, P.; Healy, E.; Jackson, I.; Rees, J.L.; Thody, A.J. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat. Genet. 1995, 11, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Makova, K.; Norton, H. Worldwide polymorphism at the MC1R locus and normal pigmentation variation in humans. Peptides 2005, 26, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Lamason, R.L.; Mohideen, M.A.; Mest, J.R.; Wong, A.C.; Norton, H.L.; Aros, M.C.; Jurynec, M.J.; Mao, X.; Humphreville, V.R.; Humbert, J.E.; et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 2005, 310, 1782–1786. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, I.; Harihara, S.; Jin, F.; Nishimukai, H.; Fujihara, J.; Fukumori, Y.; Takeshita, H.; Umetsu, K.; Saitou, N. Distribution of OCA2 *481Thr and OCA2 *615Arg, associated with hypopigmentation, in several additional populations. Leg. Med. 2011, 13, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, I.; Umetsu, K.; Harihara, S.; Miyoshi, A.; Saitou, N.; Park, K.S.; Dashnyam, B.; Jin, F.; Lucotte, G.; Chattopadhyay, P.K.; et al. OCA2 481Thr, a hypofunctional allele in pigmentation, is characteristic of northeastern Asian populations. J. Hum. Genet. 2007, 52, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Eaton, K.; Edwards, M.; Krithika, S.; Cook, G.; Norton, H.; Parra, E.J. Association study confirms the role of two OCA2 polymorphisms in normal skin pigmentation variation in East Asian populations. Am. J. Hum. Biol. 2015, 27, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Kanetsky, P.A.; Swoyer, J.; Panossian, S.; Holmes, R.; Guerry, D.; Rebbeck, T.R. A polymorphism in the agouti signaling protein gene is associated with human pigmentation. Am. J. Hum. Genet. 2002, 70, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.C.; Wollstein, A.; Lao, O.; Hofman, A.; Klaver, C.C.; Uitterlinden, A.G.; Nijsten, T.; Kayser, M.; Liu, F. Comprehensive candidate gene study highlights UGT1A and BNC2 as new genes determining continuous skin color variation in Europeans. Hum. Genet. 2013, 132, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.; Kraft, P.; Qureshi, A.A.; Guo, Q.; Chen, C.; Hankinson, S.E.; Hu, F.B.; Thomas, G.; Hoover, R.N.; Chanock, S.; et al. Genome-wide association study of tanning phenotype in a population of European ancestry. J. Investig. Dermatol. 2009, 129, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.L.; Iles, M.M.; Glass, D.; Zhu, G.; Barrett, J.H.; Höiom, V.; Zhao, Z.Z.; Sturm, R.A.; Soranzo, N.; Hammond, C.; et al. IRF4 variants have age-specific effects on nevus count and predispose to melanoma. Am. J. Hum. Genet. 2010, 87, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, C.; Grill, C.; Stacey, S.N.; Metcalf, A.M.; Gorkin, D.U.; Robinson, K.C.; Van Otterloo, E.; Kim, R.S.; Bergsteinsdottir, K.; Ogmundsdottir, M.H.; et al. A polymorphism in IRF4 affects human pigmentation through a tyrosinase-dependent MITF/TFAP2A pathway. Cell 2013, 155, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Moskvina, V.; Smith, M.; Ivanov, D.; Blackwood, D.; StClair, D.; Hultman, C.; Toncheva, D.; Gill, M.; Corvin, A.; O’Dushlaine, C.; et al. Genetic differences between five European populations. Hum. Hered. 2010, 70, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Izagirre, N.; Smith-Zubiaga, I.; Gardeazabal, J.; Díaz-Ramón, J.L.; Díaz-Pérez, J.L.; Zelenika, D.; Boyano, M.D.; Smit, N.; de la Rúa, C. Complex signatures of selection for the melanogenic loci TYR, TYRP1 and DCT in humans. BMC Evol. Biol. 2008, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, N.G.; Chaplin, G. The evolution of human skin coloration. J. Hum. Evol. 2000, 39, 57–106. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, N.G. The evolution of human skin and skin color. Annu. Rev. Anthropol. 2004, 33, 585–623. [Google Scholar] [CrossRef]
- Rogers, A.R.; Iltis, D.; Wooding, S. Genetic variation at the MC1R locus and the time since loss of body hair. Curr. Anthropol. 2004, 45, 105–108. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. Population differences of two coding SNPs in pigmentation-related genes SLC24A5 and SLC45A. Int. J. Legal Med. 2007, 121, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Harding, R.M.; Healy, E.; Ray, A.J.; Ellis, N.S.; Flanagan, N.; Todd, C.; Dixon, C.; Sajantila, A.; Jackson, I.J.; Birch-Machin, M.A.; et al. Evidence for variable selective pressures at MC1R. Am. J. Hum. Genet. 2000, 66, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.C.; Heckman, C.J.; Kloss, J.D.; Manne, S.L. Comparing alternative methods of measuring skin color and damage. Cancer Causes Control 2009, 20, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ravnbak, M.H. Objective determination of Fitzpatrick skin type. Dan. Med. Bull. 2010, 57, B4153. [Google Scholar] [PubMed]
- Pichon, L.C.; Landrine, H.; Corral, I.; Hao, Y.; Mayer, J.A.; Hoerster, K.D. Measuring skin cancer risk in African Americans: Is the Fitzpatrick Skin Type Classification Scale culturally sensitive? Ethn. Dis. 2010, 20, 174–179. [Google Scholar] [PubMed]
- Galindo, G.R.; Mayer, J.A.; Slymen, D.; Almaguer, D.D.; Clapp, E.; Pichon, L.C.; Hoerster, K.; Elder, J.P. Sun sensitivity in 5 US ethnoracial groups. Cutis 2007, 80, 25–30. [Google Scholar] [PubMed]
- Choe, Y.B.; Jang, S.J.; Jo, S.J.; Ahn, K.J.; Youn, J.I. The difference between the constitutive and facultative skin color does not reflect skin phototype in Asian skin. Skin Res. Technol. 2006, 12, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.I.; Oh, J.K.; Kim, B.K.; Suh, D.H.; Chung, J.H.; Oh, S.J.; Kim, J.J.; Kang, S.H. Relationship between skin phototype and MED in Korean, brown skin. Photodermatol. Photoimmunol. Photomed. 1997, 13, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Leenutaphong, V.; Jiamton, S. UVB phototherapy for pityriasis rosea: A bilateral comparison study. J. Am. Acad. Dermatol. 1995, 33, 996–999. [Google Scholar] [CrossRef]
- Venkataram, M.N.; Haitham, A.A. Correlating skin phototype and minimum erythema dose in Arab skin. Int. J. Dermatol. 2003, 42, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Pershing, L.K.; Tirumala, V.P.; Nelson, J.L.; Corlett, J.L.; Lin, A.G.; Meyer, L.J.; Leachman, S.A. Reflectance spectrophotometer: The dermatologists’ sphygmomanometer for skin phototyping? J. Investig. Dermatol. 2008, 128, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Del Bino, S.; Sok, J.; Bessac, E.; Bernerd, F. Relationship between skin response to ultraviolet exposure and skin color type. Pigm. Cell Res. 2006, 19, 606–614. [Google Scholar] [CrossRef] [PubMed]
- de Rigal, J.; Abella, M.L.; Giron, F.; Caisey, L.; Lefebvre, M.A. Development and validation of a new Skin Color Chart. Skin Res. Technol. 2007, 13, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.K.; Robinson, J.K. Color bar tool for skin type self-identification: A cross-sectional study. J. Am. Acad. Dermatol. 2015, 73, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Chardon, A.; Cretois, I.; Hourseau, C. Skin colour typology and suntanning pathways. Int. J. Cosm. Sc. 1991, 13, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, I.L.; Coombs, B.D. Skin color measurements in terms of CIELAB color space values. J. Investig. Dermatol. 1992, 99, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Del Bino, S.; Bernerd, F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br. J. Dermatol. 2013, 169 (Suppl. 3), 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hourblin, V.; Nouveau, S.; Roy, N.; de Lacharriere, O. Skin complexion and pigmentary disorders in facial skin of 1204 women in 4 Indian cities. Indian J. Dermatol. Venereol. Leprol. 2014, 80, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Del Bino, S.; Ito, S.; Sok, J.; Nakanishi, Y.; Bastien, P.; Wakamatsu, K.; Bernerd, F. Chemical analysis of constitutive pigmentation of human epidermis reveals constant eumelanin to pheomelanin ratio. Pigment Cell Melanoma Res. 2015, 28, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Hurbain, I.; Romao, M.; Sextius, P.; Bourreau, E.; Marchal, C.; Bernerd, F.; Duval, C.; Raposo, G. Melanosome Distribution in Keratinocytes in Different Skin Types: Melanosome Clusters Are Not Degradative Organelles. J. Investig. Dermatol. 2018, 138, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L.; Oliver, R.J.; Farr, P.M. A portable instrument for quantifying erythema induced by ultraviolet radiation. Br. J. Dermatol. 1984, 111, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Kollias, N.; Sayre, R.M.; Zeise, L.; Chedekel, M.R. Photoprotection by melanin. J. Photochem. Photobiol. B 1991, 9, 135–160. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Zmudzka, B.Z.; Kollias, N.; Beer, J.Z. Non-invasive measurements of skin pigmentation in situ. Pigment Cell Res. 2004, 17, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Shriver, M.D.; Parra, E.J.; Dios, S.; Bonilla, C.; Norton, H.; Jovel, C.; Pfaff, C.; Jones, C.; Massac, A.; Cameron, N.; et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Hum. Genet. 2003, 112, 387–399. [Google Scholar] [PubMed]
- Candille, S.I.; Absher, D.M.; Beleza, S.; Bauchet, M.; McEvoy, B.; Garrison, N.A.; Li, J.Z.; Myers, R.M.; Barsh, G.S.; Tang, H.; et al. Genome-wide association studies of quantitatively measured skin, hair, and eye pigmentation in four European populations. PLoS ONE 2012, 7, e48294. [Google Scholar] [CrossRef] [PubMed]
- Beleza, S.; Johnson, N.A.; Candille, S.I.; Absher, D.M.; Coram, M.A.; Lopes, J.; Campos, J.; Araújo, I.I.; Anderson, T.M.; Vilhjálmsson, B.J.; et al. Genetic architecture of skin and eye color in an African-European admixed population. PLoS Genet. 2013, 9, e1003372. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Nizammuddin, S.; Mallick, C.B.; Singh, S.; Prakash, S.; Siddiqui, N.A.; Rai, N.; Carlus, S.J.; Sudhakar, D.V.; Tripathi, V.P.; et al. Genotype-Phenotype Study of the Middle Gangetic Plain in India Shows Association of rs2470102 with Skin Pigmentation. J. Investig. Dermatol. 2017, 137, 670–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Wen, B.; Kayser, M. Colorful DNA polymorphisms in humans. Semin. Cell Dev. Biol. 2013, 24, 562–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokowski, R.P.; Pant, P.V.; Dadd, T.; Fereday, A.; Hinds, D.A.; Jarman, C.; Filsell, W.; Ginger, R.S.; Green, M.R.; van der Ouderaa, F.J.; et al. A genomewide association study of skin pigmentation in a South Asian population. Am. J. Hum. Genet. 2007, 81, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Palstra, R.J.; Kayser, M. Human skin color is influenced by an intergenic DNA polymorphism regulating transcription of the nearby BNC2 pigmentation gene. Hum. Mol. Genet. 2014, 23, 5750–5762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, C.; ter Huurne, J.; Berkhout, M.; Gruis, N.; Bastiaens, M.; Bergman, W.; Willemze, R.; Bavinck, J.N. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J. Investig. Dermatol. 2001, 117, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Healy, E.; Siddiqui, S.; Flanagan, N.; Steijlen, P.M.; Rosdahl, I.; Jacques, J.P.; Rogers, S.; Turner, R.; Jackson, I.J.; et al. Melanocortin 1 receptor variants in an Irish population. J. Investig. Dermatol. 1998, 111, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Shriver, M.D.; Parra, E.J. Comparison of narrow-band reflectance spectroscopy and tristimulus colorimetry for measurements of skin and hair color in persons of different biological ancestry. Am. J. Phys. Anthropol. 2000, 112, 17–27. [Google Scholar] [CrossRef]
- Wilkes, M.; Wright, C.Y.; du Plessis, J.L.; Reeder, A. Fitzpatrick Skin Type, Individual Typology Angle, and Melanin Index in an African Population: Steps Toward Universally Applicable Skin Photosensitivity Assessments. JAMA Dermatol. 2015, 151, 902–903. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, A.; Oh, C.; Diffey, B.; Wakamatsu, K.; Ito, S.; Rees, J. Eumelanin and pheomelanin concentrations in human epidermis before and after UVB irradiation. Pigment Cell Res. 2005, 18, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.; Kyne, S.; Ito, S.; Wakamatsu, K.; Todd, C.; Thody, A. Eumelanin and phaeomelanin contents of human epidermis and cultured melanocytes. Pigment Cell Res. 1995, 8, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Kobayashi, N.; Zmudzka, B.Z.; Ito, S.; Wakamatsu, K.; Yamaguchi, Y.; Korossy, K.S.; Miller, S.A.; Beer, J.Z.; Hearing, V.J. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 2003, 17, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Thody, A.J.; Higgins, E.M.; Wakamatsu, K.; Ito, S.; Burchill, S.A.; Marks, J.M. Pheomelanin as well as eumelanin is present in human epidermis. J. Investig. Dermatol. 1991, 97, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.; Quinn, A.G.; Ito, S.; Thody, A.J. The presence of tyrosinase and related proteins in human epidermis and their relationship to melanin type. Pigment Cell Res. 1994, 7, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, H.; Ito, S.; Wakamatsu, K.; Thody, A.J. Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment Cell Res. 1996, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Fujita, K. Microanalysis of eumelanin and pheomelanin in hair and melanomas by chemical degradation and liquid chromatography. Anal. Biochem. 1985, 144, 527–536. [Google Scholar] [CrossRef]
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: Application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 2011, 24, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Ito, S.; Rees, J.L. The usefulness of 4-amino-3-hydroxyphenylalanine as a specific marker of pheomelanin. Pigment Cell Res. 2002, 15, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Godechal, Q.; Ghanem, G.E.; Cook, M.G.; Gallez, B. Electron paramagnetic resonance spectrometry and imaging in melanomas: Comparison between pigmented and nonpigmented human malignant melanomas. Mol. Imaging 2013, 12, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Babiarz-Magee, L.; Chen, N.; Seiberg, M.; Lin, C.B. The expression and activation of protease-activated receptor-2 correlate with skin color. Pigment Cell Res. 2004, 17, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, G.; Bolasco, G.; Aspite, N.; Lucania, G.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. J. Investig. Dermatol. 2008, 128, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Gerald, A.B.; Pathak, M.A.; Fitzpatrick, T.B. Racial differences in the fate of melanosomes in human epidermis. Nature 1969, 222, 1081–1082. [Google Scholar] [CrossRef] [PubMed]
- Konrad, K.; Wolff, K. Hyperpigmentation, melanosome size, and distribution patterns of melanosomes. Arch. Dermatol. 1973, 107, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Thong, H.Y.; Jee, S.H.; Sun, C.C.; Boissy, R.E. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br. J. Dermatol. 2003, 149, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, A.K.; Bolasco, G.; Correia, M.S.; Pereira, F.J.C.; Iannone, L.; Hume, A.N.; Kirkpatrick, N.; Picardo, M.; Torrisi, M.R.; Rodrigues, I.P.; et al. Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J. Investig. Dermatol. 2014, 134, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.S.; Moreiras, H.; Pereira, F.J.C.; Neto, M.V.; Festas, T.C.; Tarafder, A.K.; Ramalho, J.S.; Seabra, M.C.; Barral, D.C. Melanin Transferred to Keratinocytes Resides in Nondegradative Endocytic Compartments. J. Investig. Dermatol. 2018, 138, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Minwalla, L.; Zhao, Y.; Le, P.I.; Wickett, R.R.; Boissy, R.E. Keratinocytes play a role in regulating distribution patterns of recipient melanosomes in vitro. J. Investig. Dermatol. 2001, 117, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Hachiya, A.; Sriwiriyanont, P.; Ohuchi, A.; Kitahara, T.; Takema, Y.; Visscher, M.O.; Boissy, R.E. Functional analysis of keratinocytes in skin color using a human skin substitute model composed of cells derived from different skin pigmentation types. FASEB J. 2007, 21, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Koshoffer, A.; Wickett, R.R.; Schwemberger, S.; Babcock, G.; Hakozaki, T.; Boissy, R.E. Epidermal keratinocytes from light vs. dark skin exhibit differential degradation of melanosomes. J. Investig. Dermatol. 2011, 131, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Murase, D.; Hachiya, A.; Takano, K.; Hicks, R.; Visscher, MO.; Kitahara, T.; Hase, T.; Takema, Y.; Yoshimori, T. Autophagy has a significant role in determining skin color by regulating melanosome degradation in keratinocytes. J. Investig. Dermatol. 2013, 133, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Koshoffer, A.; Wickett, R.R.; Hakozaki, T.; Boissy, R.E. Hydrolytic enzymes of the interfollicular epidermis differ in expression and correlate with the phenotypic difference observed between light and dark skin. J. Dermatol. 2013, 40, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Seiberg, M.; Lin, C.B. Cathepsin L2 levels inversely correlate with skin color. J. Investig. Dermatol. 2006, 126, 2345–2347. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004, 17, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Bastonini, E.; Kovacs, D.; Picardo, M. Skin Pigmentation and Pigmentary Disorders: Focus on Epidermal/Dermal Cross-Talk. Ann. Dermatol. 2016, 28, 279–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Hearing, V.J. Physiological factors that regulate skin pigmentation. Biofactors 2009, 35, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadono, S.; Manaka, I.; Kawashima, M.; Kobayashi, T.; Imokawa, G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J. Investig. Dermatol. 2001, 116, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Coelho, S.G.; Ebsen, D.; Smuda, C.; Mahns, A.; Miller, S.A.; Beer, J.Z.; Kolbe, L.; Hearing, V.J. Epidermal gene expression and ethnic pigmentation variations among individuals of Asian, European and African ancestry. Exp. Dermatol. 2014, 23, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Seiberg, M.; Paine, C.; Sharlow, E.; Andrade-Gordon, P.; Costanzo, M.; Eisinger, M.; Shapiro, S.S. Inhibition of melanosome transfer results in skin lightening. J. Investig. Dermatol. 2000, 115, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Yoshida-Amano, Y.; Hachiya, A.; Ohuchi, A.; Kobinger, G.P.; Kitahara, T.; Takema, Y.; Fukuda, M. Essential role of RAB27A in determining constitutive human skin color. PLoS ONE 2012, 7, e41160. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Cohen, C.; Chagnoleau, C.; Flouret, V.; Bourreau, E.; Bernerd, F. Key regulatory role of dermal fibroblasts in pigmentation as demonstrated using a reconstructed skin model: Impact of photo-aging. PLoS ONE 2014, 9, e114182. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Hu, Y.; Li, W.H.; Eisinger, M.; Seiberg, M.; Lin, C.B. The role of keratinocyte growth factor in melanogenesis: A possible mechanism for the initiation of solar lentigines. Exp. Dermatol. 2010, 19, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.B.; Hu, Y.; Rossetti, D.; Chen, N.; David, C.; Slominski, A.; Seiberg, M. Immuno-histochemical evaluation of solar lentigines: The association of KGF/KGFR and other factors with lesion development. J. Dermatol. Sci. 2010, 59, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Yoshimura, K.; Suzuki, Y.; Uchida, G.; Kitano, Y.; Harii, K.; Imokawa, G. The mechanism of epidermal hyperpigmentation in cafe-au-lait macules of neurofibromatosis type 1 (von Recklinghausen’s disease) may be associated with dermal fibroblast-derived stem cell factor and hepatocyte growth factor. Br. J. Dermatol. 2003, 148, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Itami, S.; Watabe, H.; Yasumoto, K.; Abdel-Malek, Z.A.; Kubo, T.; Rouzaud, F.; Tanemura, A.; Yoshikawa, K.; Hearing, V.J. Mesenchymal-epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J. Cell Biol. 2004, 165, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Morita, A.; Maeda, A.; Hearing, V.J. Regulation of skin pigmentation and thickness by Dickkopf 1 (DKK1). J. Investig. Dermatol. Symp. Proc. 2009, 14, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Wolber, R.; Gerwat, W.; Mann, T.; Batzer, J.; Smuda, C.; Liu, H.; Kolbe, L.; Hearing, V.J. The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J. Cell Sci. 2010, 123, 3102–3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.; Kolbe, L.; Hearing, V.J. Characterization of the bioactive motif of neuregulin-1, a fibroblast-derived paracrine factor that regulates the constitutive color and the function of melanocytes in human skin. Pigment Cell Melanoma Res. 2012, 25, 477–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, M.; Raposo, G. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, R.; Poy, M.N.; Stoffel, M.; Fuchs, E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature 2008, 452, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, S.; Kumazaki, M.; Yasui, Y.; Mori, T.; Yamada, N.; Akao, Y. MicroRNA-203 regulates melanosome transport and tyrosinase expression in melanoma cells by targeting kinesin superfamily protein 5b. J. Investig. Dermatol. 2014, 134, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Dynoodt, P.; Mestdagh, P.; Van, P.G.; Vandesompele, J.; Goossens, K.; Peelman, L.J.; Geusens, B.; Speeckaert, R.M.; Lambert, J.L.; Van Gele, M.J. Identification of miR-145 as a key regulator of the pigmentary process. J. Investig. Dermatol. 2013, 133, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Choi, S.H.; Kim, C.H.; Lee, C.H.; Lee, T.R.; Lee, A.Y. Reduced miR-675 in exosome in H19 RNA-related melanogenesis via MITF as a direct target. J. Investig. Dermatol. 2014, 134, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, H.; Xue, L.; Dong, Y.; Yang, L.; Fan, R.; Yu, X.; Tian, X.; Ma, S.; Smith, G.W. Coat color determination by miR-137 mediated down-regulation of microphthalmia-associated transcription factor in a mouse model. RNA 2012, 18, 1679–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; He, J.; Jia, X.; Jiang, J.; Bai, R.; Yu, X.; Lv, L.; Fan, R.; He, X.; Geng, J.; et al. MicroRNA-25 functions in regulation of pigmentation by targeting the transcription factor MITF in Alpaca (Lama pacos) skin melanocytes. Domest. Anim. Endocrinol. 2010, 38, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C.; Lu, Z.; Sauter, E. Vitamin D and skin physiology: A D-lightful story. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V28–V33. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.; Tuckey, R.C. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 2015, 5, 14875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, F.; Lucas, R.; de Gruijl, F.; Norval, M. A systematic review of the influence of skin pigmentation on changes in the concentrations of vitamin D and 25-hydroxyvitamin D in plasma/serum following experimental UV irradiation. Photochem. Photobiol. Sci. 2015, 14, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Chadwick, C.A.; Harrison, G.I.; Nikaido, O.; Ramsden, J.; Potten, C.S. The similarity of action spectra for thymine dimers in human epidermis and erythema suggests that DNA is the chromophore for erythema. J. Investig. Dermatol. 1998, 111, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Agin, P.P.; Desrochers, D.L.; Sayre, R.M. The relationship of immediate pigment darkening to minimal erythemal dose, skin type, and eye color. Photodermatol 1985, 2, 288–294. [Google Scholar] [PubMed]
- Andreassi, L.; Simoni, S.; Fiorini, P.; Fimiani, M. Phenotypic characters related to skin type and minimal erythemal dose. Photodermatol 1987, 4, 43–46. [Google Scholar] [PubMed]
- Ha, T.; Javedan, H.; Waterston, K.; Naysmith, L.; Rees, J.L. The relationship between constitutive pigmentation and sensitivity to ultraviolet radiation induced erythema is dose-dependent. Pigment Cell Res. 2003, 16, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Kawada, A.; Hiruma, M.; Ishibashi, A.; Arai, S. The relationship among minimal erythema dose, minimal delayed tanning dose, and skin color. J. Dermatol. 1993, 20, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.A.; Aquilina, S.; Milligan, P.J.; Walker, S.L.; Hawk, J.L.; Young, A.R. Photoadaptation during Narrowband Ultraviolet-B Therapy Is Independent of Skin Type: A Study of 352 Patients. J. Investig. Dermatol. 2006, 126, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.M.; Desrochers, D.L.; Wilson, C.J.; Marlowe, E. Skin type, minimal erythema dose (MED), and sunlight acclimatization. J. Am. Acad. Dermatol. 1981, 5, 439–443. [Google Scholar] [CrossRef]
- Shono, S.; Imura, M.; Ota, M.; Ono, S.; Toda, K. The relationship of skin color, UVB-induced erythema, and melanogenesis. J. Investig. Dermatol. 1985, 84, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Takiwaki, H.; Shirai, S.; Kohno, H.; Soh, H.; Arase, S. The degrees of UVB-induced erythema and pigmentation correlate linearly and are reduced in a parallel manner by topical anti-inflammatory agents. J. Investig. Dermatol. 1994, 103, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.I.; Young, A.R. Ultraviolet radiation-induced erythema in human skin. Methods 2002, 28, 14–19. [Google Scholar] [CrossRef]
- Dornelles, S.; Goldim, J.; Cestari, T. Determination of the minimal erythema dose and colorimetric measurements as indicators of skin sensitivity to UV-B radiation. Photochem. Photobiol. 2004, 79, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Kaidbey, K.H.; Agin, P.P.; Sayre, R.M.; Kligman, A.M. Photoprotection by melanin--a comparison of black and Caucasian skin. J. Am. Acad. Dermatol. 1979, 1, 249–260. [Google Scholar] [CrossRef]
- Montagna, W.; Prota, G.; Kenney, J.A., Jr. Black Skin Structure and Function; Academic Press: London, UK, 1993; pp. 55–60. [Google Scholar]
- Scotto, J.; Fears, T.R.; Fraumeni, J.F., Jr. Incidence of Nonmelanoma Skin Cancer in the Unites States; DHHS Report No. NIH 82-2433; U.S. Goverment Printing Office, National Cancer Institute: Washington, DC, USA, 1981.
- Phan, T.A.; Halliday, G.M.; Barnetson, R.S.; Damian, D.L. Melanin differentially protects from the initiation and progression of threshold UV-induced erythema depending on UV waveband. Photodermatol. Photoimmunol. Photomed. 2006, 22, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A.; Soter, N.A.; Stoff, J.S.; Mihm, M.C., Jr. The human sunburn reaction: Histologic and biochemical studies. J. Am. Acad. Dermatol. 1981, 5, 411–422. [Google Scholar] [CrossRef]
- Bernerd, F.; Asselineau, D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev. Biol. 1997, 183, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Pontén, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, K.H. Sunlight and skin cancer: Another link revealed. Proc. Natl. Acad. Sci. USA 1997, 94, 11–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarasin, A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutat. Res. 1999, 428, 5–10. [Google Scholar] [CrossRef]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.; Halaban, R.; Douki, T.; Brash, D.E. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.G.; Zmudzka, B.Z.; Yin, L.; Miller, S.A.; Yamaguchi, Y.; Tadokoro, T.; Hearing, V.J.; Beer, J.Z. Non-invasive diffuse reflectance measurements of cutaneous melanin content can predict human sensitivity to ultraviolet radiation. Exp. Dermatol. 2013, 22, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijken, F.; Bruijnzeel, P.L.; Van Weelden, H.; Kiekens, R.C. Responses of black and white skin to solar-simulating radiation: Differences in DNA photodamage, infiltrating neutrophils, proteolytic enzymes induced, keratinocyte activation, and IL-10 expression. J. Investig. Dermatol. 2004, 122, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Nakagawa, A.; Muramatsu, T.; Yamashina, Y.; Shirai, T.; Hashimoto, M.W.; Ishigaki, Y.; Ohnishi, T.; Mori, T. Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. J. Investig. Dermatol. 1998, 110, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M. Common links among the pathways leading to UV-induced immunosuppression. J. Investig. Dermatol. 2010, 130, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.A.; Jaenicke, K.F.; Anderson, R.R. Erythema and melanogenesis action spectra of normal human skin. Photochem. Photobiol. 1982, 36, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Takahashi, K.; Zmudzka, B.Z.; Kornhauser, A.; Miller, S.A.; Tadokoro, T.; Berens, W.; Beer, J.Z.; Hearing, V.J. Human skin responses to UV radiation: Pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006, 20, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.M.; Cragg, N.; Chadwick, C.A.; Potten, C.S.; Young, A.R. Repeated ultraviolet exposure affords the same protection against DNA photodamage and erythema in human skin types II and IV but is associated with faster DNA repair in skin type IV. J. Investig. Dermatol. 2002, 118, 825–829. [Google Scholar] [PubMed]
- Fajuyigbe, D.; Lwin, S.M.; Diffey, B.L.; Baker, R.; Tobin, D.J.; Sarkany, R.P.E.; Young, A.R. Melanin distribution in human epidermis affords localized protection against DNA photodamage and concurs with skin cancer incidence difference in extreme phototypes. FASEB J. 2018, 32, 3700–3706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norval, M.; Kellett, P.; Wright, C.Y. The incidence and body site of skin cancers in the population groups of South Africa. Photodermatol. Photoimmunol. Photomed. 2014, 30, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Del Bino, S.; Sok, J.; Bernerd, F. Assessment of ultraviolet-radiation-induced DNA damage within melanocytes in skin of different constitutive pigmentation. Br. J. Dermatol. 2013, 168, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Nouveau-Richard, S.; Yang, Z.; Mac-Mary, S.; Li, L.; Bastien, P.; Tardye, I.; Bouillon, C.; Humbert, P.; de Lacharrière, O. Skin ageing: A comparison between Chinese and European populations A pilot study. J. Dermatol. Sci. 2005, 40, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.G.; Chan, H.H. The Asian dermatologic patient: Review of common pigmentary disorders and cutaneous diseases. Am. J. Clin. Dermatol. 2009, 10, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lim, H.W. Cutaneous diseases in Asians. Dermatol. Clin. 2003, 21, 669–677. [Google Scholar] [CrossRef]
- Halder, R.M.; Nootheti, P.K. Ethnic skin disorders overview. J. Am. Acad. Dermatol. 2003, 48, S143–S148. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C. Epidemiology of skin diseases in people of color. Cutis 2003, 71, 271–275. [Google Scholar] [PubMed]
- Perez, M.; Luke, J.; Rossi, A. Melasma in Latin Americans. J. Drugs Dermatol. 2011, 10, 517–523. [Google Scholar] [PubMed]
- Ishikawa, T.; Kodama, K.; Matsumoto, J.; Takayama, S. Photoprotective role of epidermal melanin granules against ultraviolet damage and DNA repair in guinea pig skin. Cancer Res. 1984, 44, 5195–5199. [Google Scholar] [PubMed]
- Kobayashi, N.; Muramatsu, T.; Yamashina, Y.; Shirai, T.; Ohnishi, T.; Mori, T. Melanin reduces ultraviolet-induced DNA damage formation and killing rate in cultured human melanoma cells. J. Investig. Dermatol. 1993, 101, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Schothorst, A.A.; Evers, L.M.; Noz, K.C.; Filon, R.; van Zeeland, A.A. Pyrimidine dimer induction and repair in cultured human skin keratinocytes or melanocytes after irradiation with monochromatic ultraviolet radiation. J. Investig. Dermatol. 1991, 96, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Smit, N.P.; Vink, A.A.; Kolb, R.M.; Steenwinkel, M.J.; van den Berg, P.T.; van Nieuwpoort, F.; Roza, L.; Pavel, S. Melanin offers protection against induction of cyclobutane pyrimidine dimers and 6-4 photoproducts by UVB in cultured human melanocytes. Photochem. Photobiol. 2001, 74, 424–430. [Google Scholar] [CrossRef]
- Yohn, J.J.; Lyons, M.B.; Norris, D.A. Cultured human melanocytes from black and white donors have different sunlight and ultraviolet A radiation sensitivities. J. Investig. Dermatol. 1992, 99, 454–459. [Google Scholar] [CrossRef] [PubMed]
- De Winter, S.; Vink, A.A.; Roza, L.; Pavel, S. Solar-simulated skin adaptation and its effect on subsequent UV-induced epidermal DNA damage. J. Investig. Dermatol. 2001, 117, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Barnetson, R.S.; Ooi, T.K.; Zhuang, L.; Halliday, G.M.; Reid, C.M.; Walker, P.C.; Humphrey, S.M.; Kleinig, M.J. [Nle4-D-Phe7]-alpha-melanocyte-stimulating hormone significantly increased pigmentation and decreased UV damage in fair-skinned Caucasian volunteers. J. Investig. Dermatol. 2006, 126, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.E.; Kadekaro, A.L.; Kavanagh, R.J.; Wakamatsu, K.; Terzieva, S.; Schwemberger, S.; Babcock, G.; Rao, M.B.; Ito, S.; Abdel-Malek, Z.A. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigment Cell Res. 2006, 19, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Kadekaro, A.L.; Wakamatsu, K.; Ito, S.; Abdel-Malek, Z.A. Cutaneous photoprotection and melanoma susceptibility: Reaching beyond melanin content to the frontiers of DNA repair. Front. Biosci. 2006, 11, 2157–2173. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.F.; Jacques, S.L.; Stuart, M.E.; Gange, R.W. Immediate pigment darkening: Visual and reflectance spectrophotometric analysis of action spectrum. Photochem. Photobiol. 1990, 51, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochem. Photobiol. Sci. 2013, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Moyal, D.; Chardon, A.; Kollias, N. Determination of UVA protection factors using the persistent pigment darkening (PPD) as the end point. (Part 1). Calibration of the method. Photodermatol. Photoimmunol. Photomed. 2000, 16, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Moyal, D.; Chardon, A.; Kollias, N. UVA protection efficacy of sunscreens can be determined by the persistent pigment darkening (PPD) method. (Part 2). Photodermatol. Photoimmunol. Photomed. 2000, 16, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kaidbey, K.H.; Kligman, A.M. The acute effects of long-wave ultraviolet radiation on human skin. J. Investig. Dermatol. 1979, 72, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Honigsmann, H.; Schuler, G.; Aberer, W.; Romani, N.; Wolff, K. Immediate pigment darkening phenomenon. A reevaluation of its mechanisms. J. Investig. Dermatol. 1986, 87, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Routaboul, C.; Denis, A.; Vinche, A. Immediate pigment darkening: Description, kinetic and biological function. Eur. J. Dermatol. 1999, 9, 95–99. [Google Scholar] [PubMed]
- Maeda, K.; Hatao, M. Involvement of photooxidation of melanogenic precursors in prolonged pigmentation induced by ultraviolet, A. J. Investig. Dermatol. 2004, 122, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Jimbow, K.; Fitzpatrick, T.B. Changes in distribution pattern of cytoplasmic filaments in human melanocytes during ultraviolet-mediated melanin pigmentation. The role of the 100-Angstrom filaments in the elongation of melanocytic dendrites and in the movement and transfer of melanosomes. J. Cell Biol. 1975, 65, 481–488. [Google Scholar] [PubMed]
- Lavker, R.M.; Kaidbey, K.H. Redistribution of melanosomal complexes within keratinocytes following UV-A irradiation: A possible mechanism for cutaneous darkening in man. Arch. Dermatol. Res. 1982, 272, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Black, G.; Matzinger, E.; Gange, R.W. Lack of photoprotection against UVB-induced erythema by immediate pigmentation induced by 382 nm radiation. J. Investig. Dermatol. 1985, 85, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Beitner, H. Immediate pigment-darkening reaction. Photodermatol 1988, 5, 96–100. [Google Scholar] [PubMed]
- Gange, R.W. Comparison of pigment responses in human skin to UVB and UVA radiation. Prog. Clin. Biol. Res. 1988, 256, 475–485. [Google Scholar] [PubMed]
- Kollias, N.; Bykowski, J.L. Immediate pigment darkening thresholds of human skin to monochromatic (362 nm) ultraviolet A radiation are fluence rate dependent. Photodermatol. Photoimmunol. Photomed. 1999, 15, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Honigsmann, H. Erythema and pigmentation. Photodermatol. Photoimmunol. Photomed. 2002, 18, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Marionnet, C.; Nouveau, S.; Hourblin, V.; Pillai, K.; Manco, M.; Bastien, P.; Tran, C.; Tricaud, C.; de Lacharrière, O.; Bernerd, F. UVA1-Induced Skin Darkening Is Associated with Molecular Changes Even in Highly Pigmented Skin Individuals. J. Investig. Dermatol. 2017, 137, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Beitner, H.; Ringborg, U.; Wennersten, G.; Lagerlof, B. Further evidence for increased light sensitivity in patients with malignant melanoma. Br. J. Dermatol. 1981, 104, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.A.; Riley, F.C.; Fitzpatrick, T.B. Melanogenesis in human skin following exposure to long-wave ultraviolet and visible light. J. Investig. Dermatol. 1962, 39, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K.; Sarna, T. Photodegradation of Eumelanin and Pheomelanin and Its Pathophysiological Implications. Photochem. Photobiol. 2018, 94, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kikuta, M.; Koike, S.; Szewczyk, G.; Sarna, M.; Zadlo, A.; Sarna, T.; Wakamatsu, K. Roles of reactive oxygen species in UVA-induced oxidation of 5,6-dihydroxyindole-2-carboxylic acid-melanin as studied by differential spectrophotometric method. Pigment Cell Melanoma Res. 2016, 29, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Pilat, A.; Gerwat, W.; Skumatz, C.M.; Ito, M.; Kiyono, A.; Zadlo, A.; Nakanishi, Y.; Kolbe, L.; Burke, J.M.; et al. Photoaging of human retinal pigment epithelium is accompanied by oxidative modifications of its eumelanin. Pigment Cell Melanoma Res. 2013, 26, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, J.P. The effects of ultraviolet exposure on skin melanin pigmentation. J. Int. Med. Res. 1990, 18 (Suppl. 3), 8C–17C. [Google Scholar] [PubMed]
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Eller, M.S.; Gilchrest, B.A. Tanning as part of the eukaryotic SOS response. Pigment Cell Res. 2000, 13 (Suppl. 8), 94–97. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Paus, R. Does p53 regulate skin pigmentation by controlling proopiomelanocortin gene transcription? Pigment Cell Res. 2007, 20, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Skobowiat, C.; Dowdy, J.C.; Sayre, R.M.; Tuckey, R.C.; Slominski, A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: Regulation by ultraviolet radiation. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E484–E493. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.M.; Potten, C.S.; Young, A.R. Tanning in human skin types II and III offers modest photoprotection against erythema. Photochem. Photobiol. 1998, 68, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Yamaguchi, Y.; Batzer, J.; Coelho, S.G.; Zmudzka, B.Z.; Miller, S.A.; Wolber, R.; Beer, J.Z.; Hearing, V.J. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J. Investig. Dermatol. 2005, 124, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.G.; Miller, S.A.; Zmudzka, B.Z.; Beer, J.Z. Quantification of UV-induced erythema and pigmentation using computer-assisted digital image evaluation. Photochem. Photobiol. 2006, 82, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Alaluf, S.; Atkins, D.; Barrett, K.; Blount, M.; Carter, N.; Heath, A. The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Res. 2002, 15, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, Y.; Coelho, S.G.; Wolber, R.; Miller, S.A.; Wakamatsu, K.; Zmudzka, B.Z.; Ito, S.; Smuda, C.; Passeron, T.; Choi, W.; et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007, 20, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakamatsu, K.; Kavanagh, R.; Kadekaro, A.L.; Terzieva, S.; Sturm, R.A.; Leachman, S.; Abdel-Malek, Z.; Ito, S. Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Res. 2006, 19, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Kollias, N.; Baqer, A. An experimental study of the changes in pigmentation in human skin in vivo with visible and near infrared light. Photochem. Photobiol. 1984, 39, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.B.; Kaidbey, K.H.; Grove, G.L. Quantification of visible light-induced melanogenesis in human skin. Photodermatology 1988, 5, 197–200. [Google Scholar] [PubMed]
- Mahmoud, B.H.; Ruvolo, E.; Hexsel, C.L.; Liu, Y.; Owen, M.R.; Kollias, N.; Lim, H.W.; Hamzavi, I.H. Impact of long-wavelength UVA and visible light on melanocompetent skin. J. Investig. Dermatol. 2010, 130, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramaniam, R.; Roy, A.; Sharma, B.; Nagalakshmi, S. Are there mechanistic differences between ultraviolet and visible radiation induced skin pigmentation? Photochem. Photobiol. Sci. 2011, 10, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Duteil, L.; Cardot-Leccia, N.; Queille-Roussel, C.; Maubert, Y.; Harmelin, Y.; Boukari, F.; Ambrosetti, D.; Lacour, J.P.; Passeron, T. Differences in visible light-induced pigmentation according to wavelengths: A clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014, 27, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Kohli, I.; Chaowattanapanit, S.; Mohammad, T.F.; Nicholson, C.L.; Fatima, S.; Jacobsen, G.; Kollias, N.; Lim, H.W.; Hamzavi, I.H. Synergistic Effects of Long Wavelength Ultraviolet A1 and Visible Light on Pigmentation and Erythema. Br. J. Dermatol. 2018, 178, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Duteil, L.; Esdaile, J.; Maubert, Y.; Cathelineau, A.C.; Bouloc, A.; Queille-Roussel, C.; Passeron, T. A method to assess the protective efficacy of sunscreens against visible light-induced pigmentation. Photodermatol. Photoimmunol. Photomed. 2017, 33, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.E. Skin cancer. Melanoma and other specific nonmelanoma skin cancers. Cancer 1995, 75, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Halder, R.M.; Ara, C.J. Skin cancer and photoaging in ethnic skin. Dermatol. Clin. 2003, 21, 725–732. [Google Scholar] [CrossRef]
- Halder, R.M.; Bridgeman-Shah, S. Skin cancer in African Americans. Cancer 1995, 75, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Holman, C.D.; Armstrong, B.K. Pigmentary traits, ethnic origin, benign nevi, and family history as risk factors for cutaneous malignant melanoma. J. Natl. Cancer Inst. 1984, 72, 257–266. [Google Scholar] [PubMed]
- Katsambas, A.; Nicolaidou, E. Cutaneous malignant melanoma and sun exposure. Recent developments in epidemiology. Arch. Dermatol. 1996, 132, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Rhul, J.; Tatalovich, Z.; Mariotto, A.; et al. SEER Cancer Statistics Review, 1975-2004; National Cancer Institute: Bethesda, MD, USA, 2017.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Gloster, H.M., Jr.; Neal, K. Skin cancer in skin of color. J. Am. Acad. Dermatol. 2006, 55, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Ma, F.; Collado-Mesa, F.; Kirsner, R.S. UV radiation, latitude, and melanoma in US Hispanics and blacks. Arch. Dermatol. 2004, 140, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.; Wang, H.; Lee, J.; Chia, K.S.; Lee, H.P.; Goh, C.L. Basal cell carcinoma, squamous cell carcinoma and melanoma of the skin: Analysis of the Singapore Cancer Registry data 1968-97. Br. J. Dermatol. 2003, 148, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.Y.; Reizner, G.T.; Elpern, D.J.; Stone, J.L.; Farmer, E.R. Nonmelanoma skin cancer in Japanese ethnic Hawaiians in Kauai, Hawaii: An incidence report. J. Am. Acad. Dermatol. 1995, 33, 422–426. [Google Scholar] [CrossRef]
- Kim, M.; Boone, S.L.; West, D.P.; Rademaker, A.W.; Liu, D.; Kundu, R.V. Perception of skin cancer risk by those with ethnic skin. Arch. Dermatol. 2009, 145, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Buster, K.J.; You, Z.; Fouad, M.; Elmets, C. Skin cancer risk perceptions: A comparison across ethnicity, age, education, gender, and income. J. Am. Acad. Dermatol. 2012, 66, 771–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cestari, T.; Buster, K. Photoprotection in specific populations: Children and people of color. J. Am. Acad. Dermatol. 2017, 76, S110–S121. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, M.G.; Zadnick, J.; Deapen, D. Developing epidemic of melanoma in the Hispanic population of California. Cancer 2006, 106, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.C.; Eide, M.J.; King, J.; Saraiya, M.; Huang, Y.; Wiggins, C.; Barnholtz-Sloan, J.S.; Martin, N.; Cokkinides, V.; Miller, J.; et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J. Am. Acad. Dermatol. 2011, 65, S26–S37. [Google Scholar] [CrossRef] [PubMed]
- Shoo, B.A.; Kashani-Sabet, M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin. Cutan. Med. Surg. 2009, 28, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.A. Nonmelanoma skin cancer in persons of color. Semin. Cutan. Med. Surg. 2009, 28, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, J.; Hadjicharalambous, E.; Mehregan, D. Basal cell carcinoma in skin of color. J. Drugs Dermatol. 2012, 11, 484–486. [Google Scholar] [PubMed]
- Hu, S.; Parmet, Y.; Allen, G.; Parker, D.F.; Ma, F.; Rouhani, P.; Kirsner, R.S. Disparity in melanoma: A trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch. Dermatol. 2009, 145, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Alexis, A.F.; Obioha, J.O. Ethnicity and Aging Skin. J. Drugs Dermatol. 2017, 16, s77–s80. [Google Scholar] [PubMed]
- Vashi, N.A.; de Castro Maymone, M.B.; Kundu, R.V. Aging Differences in Ethnic Skin. J. Clin. Aesthet. Dermatol. 2016, 9, 31–38. [Google Scholar] [PubMed]
- Rawlings, A.V. Ethnic skin types: Are there differences in skin structure and function? Int. J. Cosmet. Sci. 2006, 28, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Lee, S.H.; Youn, C.S.; Park, B.J.; Kim, K.H.; Park, K.C.; Cho, K.H.; Eun, H.C. Cutaneous photodamage in Koreans: Influence of sex, sun exposure, smoking, and skin color. Arch. Dermatol. 2001, 137, 1043–1051. [Google Scholar] [PubMed]
- Chung, J.H. Photoaging in Asians. Photodermatol. Photoimmunol. Photomed. 2003, 19, 109–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.H. The effects of sunlight on the skin of Asians. In Comprehensive Series in Photomedicines; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; p. 69. [Google Scholar]
- Goh, S.H. The treatment of visible signs of senescence: The Asian experience. Br. J. Dermatol. 1990, 122 (Suppl. 35), 105–109. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Wang, Z.Q.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Maymone, M.B.C.; Neamah, H.H.; Wirya, S.A.; Patzelt, N.M.; Zancanaro, P.Q.; Vashi, N.A. Sun-protective behaviors in patients with cutaneous hyperpigmentation: A cross-sectional study. J. Am. Acad. Dermatol. 2017, 76, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.E. Management of hyperpigmentation in darker racial ethnic groups. Semin. Cutan. Med. Surg. 2009, 28, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Chua-Ty, G.; Goh, C.L.; Koh, S.L. Pattern of skin diseases at the National Skin Centre (Singapore) from 1989-1990. Int. J. Dermatol. 1992, 31, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Eun, H.C. Cutaneous photodamage in Asians. J. Dermatol. 2001, 28, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Vierkotter, A.; Kramer, U.; Sugiri, D.; Morita, A.; Yamamoto, A.; Kaneko, N.; Matsui, M.; Krutmann, J. Development of lentigines in German and Japanese women correlates with variants in the SLC45A2 gene. J. Investig. Dermatol. 2012, 132, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Cestari, T.F.; Dantas, L.P.; Boza, J.C. Acquired hyperpigmentations. An. Bras. Dermatol. 2014, 89, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamega, A.A.; Miot, L.D.; Bonfietti, C.; Gige, T.C.; Marques, M.E.; Miot, H.A. Clinical patterns and epidemiological characteristics of facial melasma in Brazilian women. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, J.P.; Arellano, I.; Berneburg, M.; Cestari, T.; Chan, H.; Grimes, P.; Hexsel, D.; Im, S.; Lim, J.; Lui, H.; et al. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.E.; Yamada, N.; Bhawan, J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am. J. Dermatopathol. 2005, 27, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.H.; Yoon, K.H.; Lee, E.S.; Kim, J.; Lee, K.B.; Yim, H.; Sohn, S.; Im, S. Melasma: Histopathological characteristics in 56 Korean patients. Br. J. Dermatol. 2002, 146, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Bahadoran, P.; Suzuki, I.; Zugaj, D.; Khemis, A.; Passeron, T.; Andres, P.; Ortonne, J.P. In vivo reflectance confocal microscopy detects pigmentary changes in melasma at a cellular level resolution. Exp. Dermatol. 2010, 19, e228–e233. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Suzuki, I.; Lee, D.J.; Ha, J.; Reiniche, P.; Aubert, J.; Deret, S.; Zugaj, D.; Voegel, J.J.; Ortonne, J.P. Transcriptional profiling shows altered expression of wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. J. Investig. Dermatol. 2011, 131, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Sarvjot, V.; Sharma, S.; Mishra, S.; Singh, A. Melasma: A clinicopathological study of 43 cases. Indian J. Pathol. Microbiol. 2009, 52, 357–359. [Google Scholar] [PubMed]
- Handel, A.C.; Miot, L.D.; Miot, H.A. Melasma: A clinical and epidemiological review. An. Bras. Dermatol. 2014, 89, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Lakhdar, H.; Zouhair, K.; Khadir, K.; Essari, A.; Richard, A.; Seité, S.; Rougier, A. Evaluation of the effectiveness of a broad-spectrum sunscreen in the prevention of chloasma in pregnant women. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Boukari, F.; Jourdan, E.; Fontas; Montaudié, H.; Castela, E.; Lacour, J.P.; Passeron, T. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: A prospective randomized comparative trial. J. Am. Acad. Dermatol. 2015, 72, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Hwang, Y.J.; Lee, S.K.; Park, K.C. Heterogeneous Pathology of Melasma and Its Clinical Implications. Int. J. Mol. Sci. 2016, 17, 824. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Picardo, M. Melasma, a photoaging disorder. Pigment Cell Melanoma Res. 2018, 31, 461–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Park, J.H.; Kim, S.J.; Kwon, J.E.; Kang, H.Y.; Lee, E.S.; Kim, Y.C. Two histopathological patterns of postinflammatory hyperpigmentation: Epidermal and dermal. J. Cutan. Pathol. 2017, 44, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Callender, V.D. Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J. Clin. Aesthet. Dermatol. 2010, 3, 20–31. [Google Scholar] [PubMed]
- Sanchez, M.R. Cutaneous diseases in Latinos. Dermatol. Clin. 2003, 21, 689–697. [Google Scholar] [CrossRef]
- Dunwell, P.; Rose, A. Study of the skin disease spectrum occurring in an Afro-Caribbean population. Int. J. Dermatol. 2003, 42, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Lacz, N.L.; Vafaie, J.; Kihiczak, N.I.; Schwartz, R.A. Postinflammatory hyperpigmentation: A common but troubling condition. Int. J. Dermatol. 2004, 43, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Abad-Casintahan, F.; Chow, S.K.; Goh, C.L.; Kubba, R.; Hayashi, N.; Noppakun, N.; See, J.; Suh, D.H.; Xiang, L.H.; Kang, S.; Asian Acne Board. Frequency and characteristics of acne-related post-inflammatory hyperpigmentation. J. Dermatol. 2016, 43, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Callender, V.D.; Alexis, A.F.; Daniels, S.R.; Kawata, AK.; Burk, C.T.; Wilcox, T.K.; Taylor, S.C. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J. Clin. Aesthet. Dermatol. 2014, 7, 19–31. [Google Scholar] [PubMed]
- Kaufman, B.P.; Aman, T.; Alexis, A.F. Postinflammatory Hyperpigmentation: Epidemiology, Clinical Presentation, Pathogenesis and Treatment. Am. J. Clin. Dermatol. 2018, 19, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K.; Akita, H.; Tanaka, S.; Yokoyama, Y.; Wakamatsu, S.; Matsunaga, K. Comparative study of treatment efficacy and the incidence of post-inflammatory hyperpigmentation with different degrees of irradiation using two different quality-switched lasers for removing solar lentigines on Asian skin. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Na, J.I.; Lee, J.H.; Roh, M.R.; Ko, J.Y.; Chang, S.E. Postinflammatory hyperpigmentation associated with treatment of solar lentigines using a Q-Switched 532-nm Nd: YAG laser: A multicenter survey. J. Dermatolog. Treat. 2017, 28, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.H.; Manstein, D.; Yu, C.S.; Shek, S.; Kono, T.; Wei, W.I. The prevalence and risk factors of post-inflammatory hyperpigmentation after fractional resurfacing in Asians. Lasers Surg. Med. 2007, 39, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Chapas, A.M.; Brightman, L.; Sukal, S.; Hale, E.; Daniel, D.; Bernstein, L.J.; Geronemus, R.G. Successful treatment of acneiform scarring with CO2 ablative fractional resurfacing. Lasers Surg Med. 2008, 40, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Manuskiatti, W.; Triwongwaranat, D.; Varothai, S.; Eimpunth, S.; Wanitphakdeedecha, R. Efficacy and safety of a carbon-dioxide ablative fractional resurfacing device for treatment of atrophic acne scars in Asians. J. Am. Acad. Dermatol. 2010, 63, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Dadzie, O.E.; Galadari, H. Chemical peeling in ethnic skin: An update. Br. J. Dermatol. 2013, 169 (Suppl. 3), 82–90. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Yadav, S.; Sarangal, R. Microneedling for acne scars in Asian skin type: An effective low cost treatment modality. J. Cosmet. Dermatol. 2014, 13, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Nouveau, S.; Duval, C.; Cardot-Leccia, N.; Piffaut, V.; Bourreau, E.; Queille-Roussel, C.; Bernerd, F. Development and validation of a reproducible model for studying post-inflammatory hyperpigmentation. Pigment Cell Melanoma Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Alexis, A.F.; Sergay, A.B.; Taylor, S.C. Common dermatologic disorders in skin of color: A comparative practice survey. Cutis 2007, 80, 387–394. [Google Scholar] [PubMed]
- Hafner, C.; Vogt, T. Seborrheic keratosis. J. Dtsch. Dermatol. Ges. 2008, 6, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, G.; Thappa, D.M.; Jaisankar, T.J.; Chandrashekar, L. Spectrum of seborrheic keratoses in South Indians: A clinical and dermoscopic study. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 483–488. [Google Scholar] [PubMed]
- Braun, R.P.; Ludwig, S.; Marghoob, A.A. Differential Diagnosis of Seborrheic Keratosis: Clinical and Dermoscopic Features. J. Drugs Dermatol. 2017, 16, 835–842. [Google Scholar] [PubMed]
- Hafner, C.; Toll, A.; Fernandez-Casado, A.; Earl, J.; Marqués, M.; Acquadro, F.; Méndez-Pertuz, M.; Urioste, M.; Malats, N.; Burns, J.E.; et al. Multiple oncogenic mutations and clonal relationship in spatially distinct benign human epidermal tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 20780–20785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidenreich, B.; Denisova, E.; Rachakonda, S.; Sanmartin, O.; Dereani, T.; Hosen, I.; Nagore, E.; Kumar, R. Genetic alterations in seborrheic keratoses. Oncotarget 2017, 8, 36639–36649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham-Brown, R.A.; Berth-Jones, J.; Dure-Smith, B.; Naafs, B.; Pembroke, A.C.; Harth, W.; Gollnick, H.; Orfanos, C.; Kurwa, A.; Bowry, V. Dermatologic problems for immigrant communities in a Western environment. Int. J. Dermatol. 1990, 29, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Noiles, K.; Vender, R. Are all seborrheic keratoses benign? Review of the typical lesion and its variants. J. Cutan. Med. Surg. 2008, 12, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, J.M.; Kilkenny, M.; Marks, R. The prevalence of seborrhoeic keratoses in an Australian population: Does exposure to sunlight play a part in their frequency? Br. J. Dermatol. 1997, 137, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Hwang, E.J.; Bae, J.H.; Park, H.E.; Lee, J.C.; Youn, J.I.; Chung, J.H. Seborrheic keratosis in the Korean males: Causative role of sunlight. Photodermatol. Photoimmunol. Photomed. 2003, 19, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; Bajdik, C.D.; Willemze, R.; De Gruijl, F.R.; Bouwes Bavinck, J.N. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J. Investig. Dermatol. 2003, 120, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.; Dorevitch, A.; Marks, R. The prevalence of seborrheic keratoses in people aged 15 to 30 years: Is the term senile keratosis redundant? Arch. Dermatol. 2000, 136, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Cario-Andre, M.; Lepreux, S.; Pain, C.; Nizard, C.; Noblesse, E.; Taieb, A. Perilesional vs. lesional skin changes in senile lentigo. J. Cutan. Pathol. 2004, 31, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Yin, L.; Smuda, C.; Batzer, J.; Hearing, V.J.; Kolbe, L. Molecular and histological characterization of age spots. Exp. Dermatol. 2017, 26, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrick, E.; Duval, C.; Nouveau, S.; Bastien, P.; Piffaut, V.; Chalmond, B.; Ortonne, J.P.; de Lacharrière, O.; Bernerd, F. Morphological and molecular characterization of actinic lentigos reveals alterations of the dermal extracellular matrix. Br. J. Dermatol. 2017, 177, 1619–1632. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Moro, O.; Tagami, H.; Kishimoto, J. Gene expression profiling analysis of solar lentigo in relation to immunohistochemical characteristics. Br. J. Dermatol. 2007, 156, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, M.T.; Westendorp, R.G.; Vermeer, B.J.; Bavinck, J.N. Ephelides are more related to pigmentary constitutional host factors than solar lentigines. Pigment Cell Res. 1999, 12, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, M.; Hoefnagel, J.; Westendorp, R.; Vermeer, B.J.; Bouwes Bavinck, J.N. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004, 17, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, K.; Mauger, E.; Latreille, J.; Jdid, R.; Malvy, D.; Gruber, F.; Galan, P.; Hercberg, S.; Tschachler, E.; Guinot, C. Freckles and solar lentigines have different risk factors in Caucasian women. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e345–e356. [Google Scholar] [CrossRef] [PubMed]
- Laville, V.; Clerc, S.L.; Ezzedine, K.; Jdid, R.; Taing, L.; Labib, T.; Coulonges, C.; Ulveling, D.; Carpentier, W.; Galan, P.; et al. A genome-wide association study in Caucasian women suggests the involvement of HLA genes in the severity of facial solar lentigines. Pigment Cell Melanoma Res. 2016, 29, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Monestier, S.; Gaudy, C.; Gouvernet, J.; Richard, M.A.; Grob, J.J. Multiple senile lentigos of the face, a skin ageing pattern resulting from a life excess of intermittent sun exposure in dark-skinned caucasians: A case-control study. Br. J. Dermatol. 2006, 154, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Tschachler, E.; Morizot, F. Ethnic differences in skin aging. In Skin Aging; Gilchrest, B.A., Krutmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; p. 23. [Google Scholar]
- Jacobs, L.C.; Hamer, M.A.; Gunn, D.A.; Deelen, J.; Lall, J.S.; van Heemst, D.; Uh, H.W.; Hofman, A.; Uitterlinden, A.G.; Griffiths, C.E.M.; et al. A Genome-Wide Association Study Identifies the Skin Color Genes IRF4, MC1R, ASIP, and BNC2 Influencing Facial Pigmented Spots. J. Investig. Dermatol. 2015, 135, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.C.; Nijsten, T. Commentary on: “A genome-wide association study in Caucasian women suggests the involvement of HLA genes in the severity of facial solar lentigines” by Laville et al. 2016. Pigment Cell Melanoma Res. 2017, 30, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.M.; Munhutu, M.; Foltis, P.; Battie, C.; Verschoore, M.; Oresajo, C. Evaluation and effectiveness of photoprotection compostion(sunscreen) on subjects of skin of color. J. Am. Acad. Dermatol. 2015, 72, 215. [Google Scholar]
- Ortonne, J.P.; Passeron, T.; Srinivas, C. Pigmentary Disorders, Prevention, Treatment and Cosmetic Contributions. Basic Science for Modern Cosmetic Dermatology; Srinivas, C., Verschoore, M., Eds.; The Health Sciences Publisher: New Delhi, India, 2015; p. 75. [Google Scholar]
- Chaowattanapanit, S.; Silpa-Archa, N.; Kohli, I.; Lim, H.W.; Hamzavi, I. Postinflammatory hyperpigmentation: A comprehensive overview: Treatment options and prevention. J. Am. Acad. Dermatol. 2017, 77, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Galappatthy, P.; Rathnayake, D. Depigmenting agents. In Pigmentary disorders, Updates in Clinical Dermatology; Kumarasinghe, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; p. 261. [Google Scholar]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.H.; Kolbe, L. Inhibition of Human Tyrosinase Requires Molecular Motifs Distinctively Different from Mushroom Tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M.; Willis, I. A new formula for depigmenting human skin. Arch. Dermatol. 1975, 111, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Torok, H.; Taylor, S.; Baumann, L.; Jones, T.; Wieder, J.; Lowe, N.; Jarret, M.; Rich, P.; Pariser, D.; Tschen, E.; et al. A large 12-month extension study of an 8-week trial to evaluate the safety and efficacy of triple combination (TC) cream in melasma patients previously treated with TC cream or one of its dyads. J. Drugs Dermatol. 2005, 4, 592–597. [Google Scholar] [PubMed]
- Chan, R.; Park, K.C.; Lee, M.H.; Lee, E.S.; Chang, S.E.; Leow, Y.H.; Tay, Y.K.; Legarda-Montinola, F.; Tsai, R.Y.; Tsai, T.H.; et al. A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) compared with hydroquinone 4% cream in Asian patients with moderate to severe melasma. Br. J. Dermatol. 2008, 159, 697–703. [Google Scholar] [PubMed]
- Ortonne, J.P.; Pandya, A.G.; Lui, H.; Hexsel, D. Treatment of solar lentigines. J. Am. Acad. Dermatol. 2006, 54, S262–S271. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Smith, N.R.; Tran, B.A.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Dermal damage promoted by repeated low-level UV-A1 exposure despite tanning response in human skin. JAMA Dermatol. 2014, 150, 401–406. [Google Scholar] [CrossRef] [PubMed]






| Gene | SNP | Population | Reference |
|---|---|---|---|
| MC1R | rs1805005 G>T | Europeans | [33] |
| rs1805006 C>A | Europeans | [33] | |
| rs1805007 C>T | Europeans | [33,34] | |
| rs1805008 C>T | Europeans | [33,34] | |
| rs1805009 G>C | Europeans | [33] | |
| rs2228479 G>A | Europeans | [33] | |
| rs11547464 G>A | Europeans | [33] | |
| rs1110400 T>C | Europeans | [33] | |
| rs885479 G>A | Europeans | [33] | |
| SLC24A5 | rs1426654 G | Africans, Melanesians | [35,36] |
| East Asians, Southeast Asians | [35,36] | ||
| Americans | [35,36] | ||
| rs1426654 G>A | Europeans | [33,35,36] | |
| South and Central Asians, Middle East, Pakistani, Iran, North India | [33,35,36] | ||
| North Africa, Ethiopia, Tanzania, Botswana | [37] | ||
| rs1426654 | Eurasians | [38] | |
| rs2470102 | KoeSan (Southern Africa) | [38] | |
| SLC45A2 | rs16891982 C | Africans, Melanesians | [35] |
| East Asians, Southeast Asians | [35] | ||
| Americans | [35] | ||
| rs16891982 C>G | Europeans | [33,35] | |
| Middle East, Pakistani | [35] | ||
| North Africa, | [35] | ||
| rs26722 G>A | Asians | [33] | |
| OCA2 | rs1800407 G>A | Europeans | [33] |
| rs1667394 A | Europeans | [34] | |
| rs7495174 A | Europeans | [34] | |
| rs1800414 A>G | East Asians | [33,39,40] | |
| rs1800404 C | Africans, sub-Saharian Africans | [33,37] | |
| South Asians, East Asians | [33,37] | ||
| Australo-Melanesians | [33,37] | ||
| rs1800404 C>T | Europeans | [33,37] | |
| Asians | [33] | ||
| Americans | [33] | ||
| South Africans | [37] | ||
| ASIP | rs4911442 A>G | Europeans | [33] |
| rs6058017 A>G | Europeans | [33] | |
| rs1015362 G | Europeans | [34] | |
| rs1015362 A>G | Africans | [33] | |
| rs 6058017 G | African Americans | [41] | |
| rs 6058017 G>A | Europeans | [33] | |
| TYR | rs1042602 C>A | Europeans | [33] |
| rs 1800422 G>A | Europeans | [33] | |
| rs1126809 G>A | Europeans | [33] | |
| rs1393350 A | Europeans | [34] | |
| TYRP1 | rs1408799 C>T | Europeans | [33] |
| rs2733832 C>T | Europeans | [33] | |
| rs34803545 | KoeSan (South Africa) | [38] | |
| KITLG | rs642742 A>G | Africans | [33] |
| rs12821256 T>C | Europeans | [33,34] | |
| IRF4 | rs12203592 C>T | Europeans | [33,42] |
| rs12203592 C | Chinese, Japanese | [42] | |
| Africans | [42] | ||
| rs1540771 G>A | Europeans | [33] | |
| SLC24A4 | rs12896399 G | Europeans | [42] |
| rs12896399 G>T | Europeans | [33,34] | |
| Asians | [33] | ||
| MFSD12 | rs56203814 C>T | Africans, East Africans | [37] |
| rs10424065 C>T | Africans, East Africans | [37] | |
| rs6510760 G | Europeans | [37] | |
| East Asians | [37] | ||
| Ethiopians, Tanzanians | [37] | ||
| rs6510760 G>A | sub-Saharians Africans | [37] | |
| South Asians | [37] | ||
| Autralo-Melanesians | [37] | ||
| rs112332856 T | Europeans | [37] | |
| East Asians | [37] | ||
| Ethiopians, Tanzanians | [37] | ||
| rs112332856 T>C | sub-Saharians Africans | [37] | |
| South Asians | [37] | ||
| Autralo-Melanesians | [37] | ||
| DDB1 | rs7120594 T | [37] | |
| rs11230664 C | East Africans | [37] | |
| South Asians | [37] | ||
| Australo-Melanesian | [37] | ||
| rs11230664 C>T | Europeans | [37] | |
| East Asians | [37] | ||
| Native Americans | [37] | ||
| TMT38 | rs7948623 A | non Africans | [37] |
| rs7948623 A>T | East Africans | [37] | |
| South Asians | [37] | ||
| Australo-Melanesian | [37] | ||
| HERC2 | rs4932620 C>T | Ethiopians, Tanzanians | [37] |
| South Asians | [37] | ||
| Austro-Melanesians | [37] | ||
| rs 6497271 A | South Asians | [37] | |
| Australo-Melanesians | [37] | ||
| Africans | [37] | ||
| rs 6497271 A>G | Europeans | [37] | |
| South Africans | [37] | ||
| SMARCA2 | rs7866411 | KoeSan (South Africa) | [38] |
| VLDLR | rs2093385 | KoeSan (South Africa) | [38] |
| SNX13 | rs2110015 >T | KoeSan (South Africa) | [38] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Bino, S.; Duval, C.; Bernerd, F. Clinical and Biological Characterization of Skin Pigmentation Diversity and Its Consequences on UV Impact. Int. J. Mol. Sci. 2018, 19, 2668. https://doi.org/10.3390/ijms19092668
Del Bino S, Duval C, Bernerd F. Clinical and Biological Characterization of Skin Pigmentation Diversity and Its Consequences on UV Impact. International Journal of Molecular Sciences. 2018; 19(9):2668. https://doi.org/10.3390/ijms19092668
Chicago/Turabian StyleDel Bino, Sandra, Christine Duval, and Françoise Bernerd. 2018. "Clinical and Biological Characterization of Skin Pigmentation Diversity and Its Consequences on UV Impact" International Journal of Molecular Sciences 19, no. 9: 2668. https://doi.org/10.3390/ijms19092668