Understanding the Heat Shock Response in the Sea Cucumber Apostichopus japonicus, Using iTRAQ-Based Proteomics
Abstract
:1. Introduction
2. Results
2.1. Overview of the Proteomics Data
| Group Name | Number |
|---|---|
| Total spectra | 272,754 |
| Spectra | 41,330 |
| Unique spectra | 38,588 |
| Peptide | 10,908 |
| Unique peptide | 10,486 |
| Protein | 3432 |
| Upregulated protein | 61 |
| Downregulated protein | 66 |

| Accession Number | Protein Description | HS vs. Control | |
|---|---|---|---|
| Mean | SD | ||
| HSPs and Related Proteins | |||
| Unigene28963 | heat shock protein 90 | 6.10 | 4.25 |
| CL6821.Contig1 | heat shock protein 70 | 1.56 | 0.62 |
| CL5625.Contig2 | heat shock protein 110 | 1.41 | 0.22 |
| Unigene15437 | heat shock protein 10 | 1.20 | 0.01 |
| CL12434.Contig1 | heat repeat-containing protein 7A | 1.36 | 0.19 |
| Detoxification and Tissue Protection | |||
| Unigene61290 | glutathione S-transferase | 1.36 | 0.07 |
| CL6008.Contig2 | glutathione S-transferase α-4-like, partial | 2.23 | 0.72 |
| Unigene25399 | sigma class glutathione S-transferase 2 | 1.29 | 0.26 |
| CL7884.Contig2 | phospholipid hydroperoxide glutathione peroxidase | 1.45 | 0.16 |
| Unigene29285 | prostaglandin D2 synthase, hematopoietic-like | 1.53 | 0.35 |
| Unigene25766 | cytochrome P450 4V2-like | 2.43 | 1.45 |
| Cell Apoptosis and Proliferation | |||
| Unigene29013 | apoptosis-inducing factor 1, mitochondrial-like, partial | 1.28 | 0.20 |
| CL4411.Contig2 | prohibitin-like | 1.19 | 0.12 |
| CL2387.Contig1 | autocrine proliferation repressor protein A-like | 3.57 | 3.08 |
| Unigene33274 | suppression of tumorigenicity 13 (colon carcinoma) (Hsp70 interacting protein) | 1.27 | 0.09 |
| CL10790.Contig2 | erlin-1 | 1.29 | 0.04 |
| Unigene35102 | mesoderm-specific transcript protein (MEST) | 2.61 | 2.19 |
| Lipid Transport and Metabolism | |||
| Unigene64084 | long-chain specific acyl-CoA dehydrogenase | 1.41 | 0.24 |
| Unigene20467 | long-chain specific acyl-CoA dehydrogenase, mitochondrial-like | 2.22 | 0.72 |
| Unigene22338 | short chain dehydrogenase/reductase family 16C, member 5-like | 1.74 | 0.58 |
| Unigene4389 | 17-β-hydroxysteroid dehydrogenase type 4 | 1.94 | 1.11 |
| Unigene5795 | 11-β-hydroxysteroid dehydrogenase | 2.54 | 1.24 |
| Unigene6420 | enoyl-CoA Hydratase family member-like | 1.62 | 0.33 |
| CL12084.Contig1 | enoyl-CoA hydratase, mitochondrial-like | 1.30 | 0.16 |
| CL759.Contig2 | hydroxyacyl-Coenzyme A dehydrogenase | 1.34 | 0.31 |
| Unigene19362 | epidermal retinol dehydrogenase 2-like | 1.97 | 0.83 |
| Unigene15259 | carnitine O-palmitoyltransferase 2, mitochondrial-like | 1.21 | 0.06 |
| Unigene11008 | non-specific lipid-transfer protein-like | 2.99 | 1.98 |
| CL4289.Contig1 | nuclear progesterone receptor | 1.46 | 0.23 |
| CL6901.Contig2 | 2′-deoxynucleoside 5′-phosphate N-hydrolase 1 | 1.43 | 0.27 |
| CL8136.Contig1 | acyl-CoA-binding protein like, ACBP2 | 2.33 | 1.46 |
| Unigene18754 | oxysterol-binding protein-related protein 9 | 1.20 | 0.18 |
| Carbohydrate Transport and Metabolism | |||
| Unigene2131 | lactase | 1.48 | 0.10 |
| Amino Acid Transport and Metabolism | |||
| CL4095.Contig2 | sphingosine-1-phosphate lyase 1 | 1.33 | 0.27 |
| Unigene23212 | branched-chain-amino-acid aminotransferase-like protein 1 | 1.39 | 0.32 |
| Energy Production and Conversion | |||
| CL10773.Contig1 | isocitrate dehydrogenase | 1.68 | 0.19 |
| Unigene18857 | electron transfer flavoprotein subunit α, mitochondrial-like | 1.27 | 0.25 |
| CL6007.Contig1 | aldehyde dehydrogenase, dimeric NADP-preferring isoform | 1.37 | 0.22 |
| Unigene22955 | α-methylacyl-CoA racemase-like | 1.65 | 0.66 |
| Unigene175 | d-glucosyl-N-acylsphingosine glucohydrolase | 1.85 | 0.31 |
| Unigene22578 | α-galactosidase | 1.91 | 1.26 |
| Unigene29260 | ATPase family AAA domain-containing protein 1 | 1.35 | 0.05 |
| CL5389.Contig1 | ATPase inhibitor, mitochondrial-like | 1.31 | 0.21 |
| Protein Synthesis | |||
| CL9215.Contig1 | aspartyl-tRNA synthetase | 1.17 | 0.11 |
| CL7807.Contig3 | RNA-binding motif protein, X chromosome | 1.25 | 0.19 |
| Unigene8195 | elongation factor Tu, mitochondrial-like | 1.34 | 0.20 |
| Others/Uncharacterized | |||
| Unigene49395 | toposome | 1.83 | 0.81 |
| Unigene28479 | natterin-3-like | 2.63 | 1.01 |
| CL6732.Contig2 | calpain-5 isoform 2 | 1.38 | 0.37 |
| Unigene22143 | phospholipase C delta isoform | 2.55 | 0.58 |
| CL1115.Contig1 | endophilin-B1-like isoform 1 | 1.32 | 0.19 |
| Unigene322 | myosin VIb-like | 1.32 | 0.24 |
| CL7807.Contig3 | atlastin-2 | 1.33 | 0.23 |
| CL9074.Contig2 | cysteine rich protein 1 | 1.59 | 0.18 |
| Unigene11767 | suppressor of G2 allele of SKP1 homolog | 1.53 | 0.14 |
| CL8638.Contig1 | development-specific protein LVN1.2 | 1.71 | 0.62 |
| Unigene22386 | uncharacterized | 1.39 | 0.35 |
| Unigene1947 | uncharacterized | 3.76 | 1.54 |
| Unigene5634 | uncharacterized | 1.66 | 0.38 |
| Unigene16247 | uncharacterized | 2.11 | 1.58 |
| Unigene62712 | uncharacterized | 11.32 | 9.13 |
| Accession Number | Protein Description | HS vs. Control | |
|---|---|---|---|
| Mean | SD | ||
| Cytoskeletal Proteins | |||
| Unigene32477 | twitchin-like | 0.67 | 0.21 |
| Unigene32260 | laminin subunit α-like | 0.68 | 0.13 |
| CL221.Contig4 | α-actinin-like | 0.76 | 0.10 |
| Unigene3881 | galectin-9-like | 0.56 | 0.29 |
| Unigene27394 | fibrillin-1-like | 0.52 | 0.17 |
| CL5005.Contig4 | cohesin subunit SA-1-like | 0.69 | 0.25 |
| Unigene9716 | titin isoform 3 | 0.72 | 0.17 |
| CL3832.Contig7 | filamin-C isoform 1 | 0.83 | 0.01 |
| Unigene30625 | muscle M-line assembly protein unc-89-like | 0.71 | 0.10 |
| Transcription and Translation | |||
| Unigene29879 | 60S ribosomal protein L8-like | 0.79 | 0.11 |
| Unigene26472 | 60S ribosomal protein L6, partial | 0.82 | 0.15 |
| Unigene8941 | ribosomal protein L4, partial | 0.82 | 0.09 |
| CL1672.Contig2 | 60S ribosomal protein L10-like | 0.73 | 0.14 |
| CL4437.Contig3 | splicing factor, proline- and glutamine-rich | 0.62 | 0.05 |
| CL5572.Contig1 | THO complex subunit 4 | 0.64 | 0.25 |
| Unigene22920 | small nuclear ribonucleoprotein-associated proteins B and B′ | 0.81 | 0.12 |
| Unigene15894 | malectin | 0.75 | 0.08 |
| DNA Replication and Repair | |||
| CL1035.Contig9 | histone H3.3 | 0.66 | 0.29 |
| Unigene5846 | histone H1-β, late embryonic | 0.61 | 0.05 |
| CL5357.Contig1 | legumain-like | 0.67 | 0.20 |
| CL64.Contig2 | poly(ADP-ribose) polymerase pme-5-like | 0.77 | 0.08 |
| Unigene9968 | ATP-binding cassette, sub-family C, member 9-like | 0.69 | 0.01 |
| Amino Acid Transport and Mechanism | |||
| CL4631.Contig1 | choline dehydrogenase, mitochondrial-like | 0.65 | 0.17 |
| Unigene11760 | branched-chain-amino-acid aminotransferase, cytosolic | 0.71 | 0.08 |
| CL3226.Contig1 | tyrosine aminotransferase-like | 0.66 | 0.28 |
| Unigene25781 | aminopeptidase N-like | 0.72 | 0.13 |
| CL12737.Contig1 | glutamyl aminopeptidase | 0.79 | 0.16 |
| CL2682.Contig1 | cytosolic serine hydroxymethyltransferase | 0.73 | 0.08 |
| CL9582.Contig2 | xaa-Pro aminopeptidase 1 isoform X3 | 0.76 | 0.09 |
| Unigene36365 | betaine homocysteine S-methyltransferase 1 | 0.58 | 0.22 |
| Unigene3911 | betaine homocysteine S-methyltransferase 1-like | 0.38 | 0.10 |
| Lipid Transport and Mechanism | |||
| Unigene27722 | peroxisomal bifunctional enzyme-like | 0.78 | 0.14 |
| Unigene10407 | peroxisomal bifunctional enzyme | 0.81 | 0.07 |
| CL8765.Contig2 | dihydropteridine reductase | 0.74 | 0.09 |
| CL1598.Contig1 | γ-butyrobetaine dioxygenase-like | 0.60 | 0.22 |
| Carbohydrate Transport and Metabolism | |||
| Unigene27857 | α-mannosidase 2C1-like | 0.47 | 0.24 |
| Unigene18547 | pyruvate carboxylase, mitochondrial | 0.73 | 0.15 |
| Hormonal and Nerve Regulation | |||
| Unigene25501 | thyroid hormone-induced protein B-like | 0.41 | 0.28 |
| CL9528.Contig2 | proactivator polypeptide | 0.72 | 0.23 |
| Unigene19435 | angiotensin-converting enzyme | 0.38 | 0.18 |
| CL7652.Contig1 | potassium channel tetramerization domain containing 6-like | 0.37 | 0.29 |
| CL5238.Contig2 | glutamate receptor 1-like | 0.68 | 0.08 |
| Others/Uncharacterized | |||
| Unigene1888b1 | sterigmatocystin biosynthesis dehydrogenase stcV | 0.65 | 0.26 |
| CL5732.Contig1 | N-acetylated-α-linked acidic dipeptidase 2-like isoform 1 | 0.58 | 0.11 |
| CL9717.Contig2 | peroxiredoxin-4-like | 0.53 | 0.07 |
| Unigene25753 | homogentisate 1,2-dioxygenase | 0.56 | 0.32 |
| CL2303.Contig2 | cathepsin L | 0.70 | 0.26 |
| Unigene8231 | α-parvin-like | 0.77 | 0.11 |
| CL7154.Contig1 | sorcin | 0.82 | 0.10 |
| Unigene32921 | thiopurine S-methyltransferase isoform X2 | 0.75 | 0.07 |
| CL2660.Contig13 | phosphatidylinositol-binding clathrin assembly protein unc-11-like isoform 6 | 0.80 | 0.09 |
| Unigene15838 | cytochrome P450 2N2 | 0.70 | 0.10 |
| CL2540.Contig2 | cytochrome P450 2U1-like | 0.58 | 0.05 |
| Unigene23131 | oocyst wall protein 4 precursor | 0.58 | 0.10 |
| CL4607.Contig1 | uterine-ovary specific-44 protein | 0.79 | 0.14 |
| CL10965.Contig2 | MAM and LDL-receptor class A domain-containing protein 2-like | 0.73 | 0.13 |
| Unigene11852 | uncharacterized | 0.74 | 0.17 |
| Unigene8201 | uncharacterized | 0.62 | 0.21 |
| Unigene63234 | uncharacterized | 0.55 | 0.34 |
| Unigene11761 | uncharacterized | 0.77 | 0.08 |
| CL11132.Contig3 | uncharacterized | 0.59 | 0.23 |
| Unigene23211 | uncharacterized | 0.77 | 0.20 |
| CL12015.Contig1 | uncharacterized | 0.58 | 0.19 |
| CL709.Contig5 | uncharacterized | 0.37 | 0.27 |
| Unigene8605 | uncharacterized | 0.78 | 0.03 |
| CL3869.Contig4 | uncharacterized | 0.58 | 0.12 |
2.2. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analyses

| Pathway ID | Pathway Term | All Proteins with Pathway Annotation (2664) | Differential Proteins with Pathway Annotation (100) | p-Value |
|---|---|---|---|---|
| Upregulated Pathways | ||||
| ko00480 | Glutathione metabolism | 30 (1.13%) | 6 (6%) | 1.37 × 10−3 |
| ko00980 | Metabolism of xenobiotics by cytochrome P450 | 46 (1.73%) | 7 (7%) | 1.51 × 10−3 |
| ko00071 | Fatty acid metabolism | 60 (2.25%) | 8 (8%) | 2.31 × 10−3 |
| ko03320 | Peroxisome proliferator-activated receptors (PPAR) signaling pathway | 64 (2.4%) | 8 (8%) | 4.35 × 10−3 |
| ko00120 | Primary bile acid biosynthesis | 56 (2.1%) | 7 (7%) | 8.82 × 10−3 |
| ko00982 | Drug metabolism-cytochrome P450 | 51 (1.91%) | 6 (6%) | 1.10 × 10−2 |
| ko05215 | Prostate cancer | 37 (1.39%) | 5 (5%) | 1.13 × 10−2 |
| Downregulated Pathways | ||||
| ko05322 | Systemic lupus erythematosus | 23 (0.86%) | 4 (4%) | 9.55 × 10−3 |
| ko04614 | Renin-angiotensin system | 51 (1.91%) | 6 (6%) | 1.10 × 10−2 |
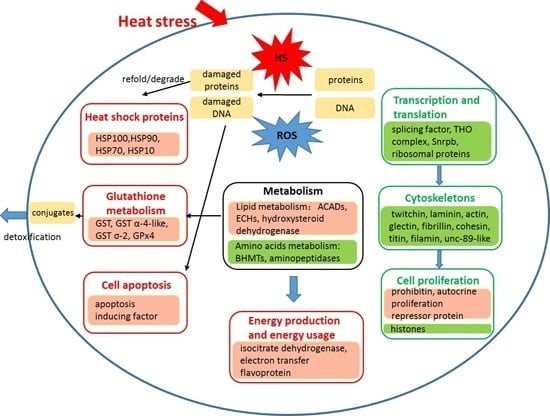
3. Discussion
3.1. Tissue Protection and Detoxification
3.2. Lipid, Amino Acid and Carbohydrate Metabolism
3.3. Energy Production and Usage
3.4. Transcription and Translation
3.5. Cell Apoptosis and Proliferation
3.6. Other Processes
4. Materials and Methods
4.1. Animals and Samples
4.2. Protein Extraction, Digestion, and iTRAQ Labeling
4.3. Fractionation by Strong Cation Exchange Chromatography (SCX) and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
4.4. Protein Identification and Quantification
4.5. GO and KEGG Pathway Enrichment Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate change 2013: The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Liao, Y. Fauna sinica: Phylum echinodermata. In Class Holothuroidea (in Chinese); Science Press: Beijing, China, 1997. [Google Scholar]
- Ji, T.; Dong, Y.; Dong, S. Growth and physiological responses in the sea cucumber, Apostichopus japonicus selenka: Aestivation and temperature. Aquaculture 2008, 283, 180–187. [Google Scholar] [CrossRef]
- Xu, D.; Sun, L.; Liu, S.; Zhang, L.; Ru, X.; Zhao, Y.; Yang, H. Molecular cloning of heat shock protein 10 (Hsp10) and 60 (Hsp60) cDNAs and their expression analysis under thermal stress in the sea cucumber Apostichopus japonicus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 171, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, H.; Zhao, H.; Chen, M.; Wang, T. The molecular characterization and expression of heat shock protein 90 (Hsp90) and 26 (Hsp26) cDNAs in sea cucumber (Apostichopus japonicus). Cell Stress Chaperones 2011, 16, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, Y.; Li, C.; Su, X.; Wang, Z.; Jin, C.; Li, Y.; Li, T. Identification of differential expressed proteins and characterization their mRNA expression in thermally stressed Apostichopus japonicus. Comp. Biochem. Physiol. D Genom. Proteom. 2013, 8, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Zieske, L.R. A perspective on the use of iTRAQ™ reagent technology for protein complex and profiling studies. J. Exp. Bot. 2006, 57, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.W.; Wang, G.; Baek, S.J.; Shen, R.-F. Comparative study of three proteomic quantitative methods, DIGE, cICAT, and iTRAQ, using 2D gel-or LC-MALDI TOF/TOF. J. Proteome Res. 2006, 5, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Karp, N.A.; Huber, W.; Sadowski, P.G.; Charles, P.D.; Hester, S.V.; Lilley, K.S. Addressing accuracy and precision issues in iTRAQ quantitation. Mol. Cell. Proteom. 2010, 9, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N. Proteomexchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, S.; Dong, Y. Parental effect of long acclimatization on thermal tolerance of juvenile sea cucumber Apostichopus japonicus. PLoS ONE 2015, 10, e0143372. [Google Scholar] [CrossRef] [PubMed]
- Parsell, D.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993, 27, 437–496. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C.; Moarefi, I.; Hartl, F.U. Hsp90: A specialized but essential protein-folding tool. J. Cell Biol. 2001, 154, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Buchner, J. Hsp90: Chaperoning signal transduction. J. Cell. Physiol. 2001, 188, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Clerico, E.M.; Tilitsky, J.M.; Meng, W.; Gierasch, L.M. How Hsp70 molecular machines interact with their substrates to mediate diverse physiological functions. J. Mol. Biol. 2015, 427, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Sun, L.; Liu, S.; Zhang, L.; Yang, H. Histological, ultrastructural and heat shock protein 70 (HSP70) responses to heat stress in the sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2015, 45, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Queitsch, C.; Hong, S.-W.; Vierling, E.; Lindquist, S. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 2000, 12, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Mogk, A.; Kummer, E.; Bukau, B. Cooperation of Hsp70 and Hsp100 chaperone machines in protein disaggregation. Front. Mol. Biosci. 2015, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Khaleque, M.A.; Sawyer, D.B.; Ciocca, D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem. Sci. 2006, 31, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Höhfeld, J.; Hartl, F.U. Role of the chaperonin cofactor Hsp10 in protein folding and sorting in yeast mitochondria. J. Cell Biol. 1994, 126, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. The effects of stress and aging on glutathione metabolism. Ageing Res. Rev. 2005, 4, 288–314. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.A.; Forman, H.J. Glutathione in defense and signaling: Lessons from a small thiol. Ann. N. Y. Acad. Sci. 2002, 973, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.L.; Bammler, T.K. Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol. Sci. 1999, 49, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Jia, F.-X.; Tian, C.-B.; Tian, Y.; Smagghe, G.; Dou, W.; Wang, J.-J. Comparative proteomic analysis of Bactrocera dorsalis (Hendel) in response to thermal stress. J. Insect Physiol. 2015, 74, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-T.; Ma, L.; Duan, W.; Wang, B.-C.; Li, J.-H.; Xu, H.-G.; Yan, X.-Q.; Yan, B.-F.; Li, S.-H.; Wang, L.-J. Differential proteomic analysis of grapevine leaves by iTRAQ reveals responses to heat stress and subsequent recovery. BMC Plant Biol. 2014, 14, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.; Kim, J.J.P. Flavoprotein structure and mechanism of action of the acyl-coa dehydrogenases. FASEB J. 1995, 9, 718–725. [Google Scholar] [PubMed]
- Bahnson, B.J.; Anderson, V.E.; Petsko, G.A. Structural mechanism of enoyl-CoA hydratase: Three atoms from a single water are added in either an E1cb stepwise or concerted fashion. Biochemistry 2002, 41, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Heunis, T.; Deane, S.; Smit, S.; Dicks, L.M. Proteomic profiling of the acid stress response in Lactobacillus plantarum 423. J. Proteome Res. 2014, 13, 4028–4039. [Google Scholar] [CrossRef] [PubMed]
- Thorne, M.A.S.; Burns, G.; Fraser, K.P.P.; Hillyard, G.; Clark, M.S. Transcription profiling of acute temperature stress in the Antarctic plunderfish Harpagifer antarcticus. Mar. Genom. 2010, 3, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Mu, H.; Zhang, H.; Chandramouli, K.H.; Qian, P.-Y.; Wong, C.K.C.; Qiu, J.-W. Understanding the regulation of estivation in a freshwater snail through iTRAQ-based comparative proteomics. J. Proteome Res. 2013, 12, 5271–5280. [Google Scholar] [CrossRef] [PubMed]
- Skiba, W.E.; Taylor, M.; Wells, M.; Mangum, J.H.; Awad, W. Human hepatic methionine biosynthesis. Purification and characterization of betaine homocysteine S-methyltransferase. J. Biol. Chem. 1982, 257, 14944–14948. [Google Scholar] [PubMed]
- Pajares, M.A.; Pérez-Sala, D. Betaine homocysteine S-methyltransferase: Just a regulator of homocysteine metabolism? Cell. Mol. Life Sci. 2006, 63, 2792–2803. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.-W.; Mehedint, M.G.; Garrow, T.A.; Zeisel, S.H. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and L-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J. Biol. Chem. 2011, 286, 36258–36267. [Google Scholar] [CrossRef] [PubMed]
- Bahl, J.J.; Matsuda, M.; DeFronzo, R.A.; Bressler, R. In vitro and in vivo suppression of gluconeogenesis by inhibition of pyruvate carboxylase. Biochem. Pharmacol. 1997, 53, 67–74. [Google Scholar] [CrossRef]
- Weidenhaupt, M.; Rossi, P.; Beck, C.; Fischer, H.M.; Hennecke, H. Bradyrhizobium japonicum possesses two discrete sets of electron transfer flavoprotein genes: fixA, fixB and etfS, etfL. Arch. Microbiol. 1996, 165, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Tomanek, L. Proteomics to study adaptations in marine organisms to environmental stress. J. Proteom. 2014, 105, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Alp, P.R.; Newsholme, E.A.; Zammit, V.A. Activities of citrate synthase and NAD+-linked and NADP+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem. J. 1976, 154, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.-H.; Son, M.-K.; Koh, H.-J.; Lee, S.-M.; Song, I.-H.; Kim, Y.-O.; Lee, Y.-S.; Jeong, K.-S.; Kim, W.B.; Park, J.-W. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. 2001, 276, 16168–16176. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.A.; Zuzow, M.J.; Tomanek, L. Proteomic responses of blue mussel (Mytilus) congeners to temperature acclimation. J. Exp. Biol. 2012, 215, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.; Campanale, J.; Foltz, K. Proteomic responses of sea urchin embryos to stressful ultraviolet radiation. Integr. Comp. Biol. 2012, 52, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wu, H.; Wei, L.; Zhao, J. ITRAQ-based quantitative proteomic analyses on the gender-specific responses in mussel Mytilus galloprovincialis to tetrabromobisphenol A. Aquat. Toxicol. 2014, 157, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Holcik, M.; Sonenberg, N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005, 6, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.R. The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 1999, 24, 437–440. [Google Scholar] [CrossRef]
- Rudra, D.; Warner, J.R. What better measure than ribosome synthesis? Genes Dev. 2004, 18, 2431–2436. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ueda, T.; Taguchi, H.; Takeuchi, N. Chaperone properties of mammalian mitochondrial translation elongation factor Tu. J. Biol. Chem. 2007, 282, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Bhadula, S.K.; Elthon, T.E.; Habben, J.E.; Helentjaris, T.G.; Jiao, S.; Ristic, Z. Heat-stress induced synthesis of chloroplast protein synthesis elongation factor (EF-Tu) in a heat-tolerant maize line. Planta 2001, 212, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Ristic, Z.; Wilson, K.; Nelsen, C.; Momcilovic, I.; Kobayashi, S.; Meeley, R.; Muszynski, M.; Habben, J. A maize mutant with decreased capacity to accumulate chloroplast protein synthesis elongation factor (EF-Tu) displays reduced tolerance to heat stress. Plant Sci. 2004, 167, 1367–1374. [Google Scholar] [CrossRef]
- Buckley, B.A.; Gracey, A.Y.; Somero, G.N. The cellular response to heat stress in the goby Gillichthys mirabilis: A cDNA microarray and protein-level analysis. J. Exp. Biol. 2006, 209, 2660–2677. [Google Scholar] [CrossRef] [PubMed]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [PubMed]
- Ikonen, E.; Fiedler, K.; Parton, R.G.; Simons, K. Prohibitin, an antiproliferative protein, is localized to mitochondria. FEBS Lett. 1995, 358, 273–277. [Google Scholar] [CrossRef]
- Brock, D.A.; Gomer, R.H. A secreted factor represses cell proliferation in dictyostelium. Development 2005, 132, 4553–4562. [Google Scholar] [CrossRef] [PubMed]
- Strahl, B.D.; Allis, C.D. The language of covalent histone modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Marushige, Y.; Marushige, K. Alterations in focal adhesion and cytoskeletal proteins during apoptosis. Anticancer Res. 1997, 18, 301–307. [Google Scholar]
- Cordeiro, O.D.; Silva, T.S.; Alves, R.N.; Costas, B.; Wulff, T.; Richard, N.; de Vareilles, M.; Conceição, L.E.; Rodrigues, P.M. Changes in liver proteome expression of senegalese sole (Solea senegalensis) in response to repeated handling stress. Mar. Biotechnol. 2012, 14, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Gunst, S.J.; Zhang, W. Actin cytoskeletal dynamics in smooth muscle: A new paradigm for the regulation of smooth muscle contraction. Am. J. Physiol. Cell Physiol. 2008, 295, C576–C587. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.P. Three muscular dystrophies: Loss of cytoskeleton-extracellular matrix linkage. Cell 1995, 80, 675–679. [Google Scholar] [CrossRef]
- Soffer, R.L. Angiotensin-converting enzyme and the regulation of vasoactive peptides. Annu. Rev. Biochem. 1976, 45, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–62. [Google Scholar] [PubMed]
- Miller, C. An overview of the potassium channel family. Genome Biol. 2000, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Briolant, S.; Almeras, L.; Belghazi, M.; Boucomont-Chapeaublanc, E.; Wurtz, N.; Fontaine, A.; Granjeaud, S.; Fusaï, T.; Rogier, C.; Pradines, B. Research Plasmodium falciparum proteome changes in response to doxycycline treatment. Malar. J. 2010, 9, 141–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gene Ontology Consortium. Available online: http://www.geneontology.org/ (accessed on 20 May 2015).
- KEGG: Kyoto Encyclopedia of Genes and Genomes. Available online: http://www.genome.jp/kegg/ (accessed on 20 May 2015).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Sun, L.; Liu, S.; Zhang, L.; Yang, H. Understanding the Heat Shock Response in the Sea Cucumber Apostichopus japonicus, Using iTRAQ-Based Proteomics. Int. J. Mol. Sci. 2016, 17, 150. https://doi.org/10.3390/ijms17020150
Xu D, Sun L, Liu S, Zhang L, Yang H. Understanding the Heat Shock Response in the Sea Cucumber Apostichopus japonicus, Using iTRAQ-Based Proteomics. International Journal of Molecular Sciences. 2016; 17(2):150. https://doi.org/10.3390/ijms17020150
Chicago/Turabian StyleXu, Dongxue, Lina Sun, Shilin Liu, Libin Zhang, and Hongsheng Yang. 2016. "Understanding the Heat Shock Response in the Sea Cucumber Apostichopus japonicus, Using iTRAQ-Based Proteomics" International Journal of Molecular Sciences 17, no. 2: 150. https://doi.org/10.3390/ijms17020150