Enhancing Photocatalytic Properties of TiO2 Photocatalyst and Heterojunctions: A Comprehensive Review of the Impact of Biphasic Systems in Aerogels and Xerogels Synthesis, Methods, and Mechanisms for Environmental Applications
Abstract
:1. Introduction
2. TiO2 as Photocatalyst and Heterojunctions
2.1. TiO2 Photocatalyst Advantages That Benefit the World Ecology and Everyday Health
2.2. Structure of TiO2 Phases: Anatase, Brookite, Rutile
Corner-Sharing and Edge-Sharing Modes of Arrangement
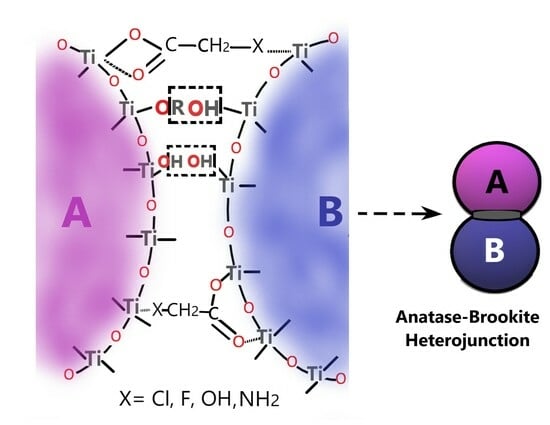
2.3. Heterophase Junctions of Anatase-Brookite (Rutile) and Photocatalyst Efficiency
2.4. TiO2 and Non-Ti Compounds; Positive Effect of Heterojunctions on Photoactivity
3. Synthesis of TiO2 Photocatalyst: A Brief Summary of Methods from the Literature
3.1. Synthesis of TiO2 Photocatalyst Containing Heterojunctions
3.2. Complex Synthesis-Principle: Sol-Gel Route
Low-Temperature- Hydrothermal Synthesis (LTHT)
3.3. Acid-Base Donors’ Effect on Complexing Gelation
3.3.1. Aging Effects on Condensation
3.3.2. Post Heat Treatments
3.3.3. Heterojunctions-Grain Boundaries
3.4. Drying Techniques Obtention of an Aerogel or Xerogel Catalyst
4. Mechanisms of Photocatalytic Activity
4.1. Influence of Physicochemical Properties on Charge Diffusion
4.2. Recombination of Charge Carriers during Photocatalytic Performance
4.3. Presence of a Scavenger in Photo-Oxidation/Reduction Mechanism
4.4. TiO2 Doping Techniques Improvement on Photo Efficiency
4.4.1. Non-Metallic Doping
4.4.2. Metallic Doping
4.4.3. Doping TiO2 with Rare Earth Elements
4.5. Doping with Metals the Role in Enhancement the Photocatalytic Activity of TiO2
5. TiO2 Photocatalyst Applications in Energy, Environment, Biomedicine Fields
5.1. Transformative Uses of Biphasic TiO2 Heterojunctions in Photocatalysis, Energy Storage, Sensors, and Catalysis
5.2. Versatile Healthcare Uses of Biphasic TiO2 Heterojunctions in Biomedicine and Beyond
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-Doped TiO2: Theory and Experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic Degradation for Environmental Applications—A Review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Su, C.Y.; Wang, L.C.; Liu, W.S.; Wang, C.C.; Perng, T.P. Photocatalysis and Hydrogen Evolution of Al- and Zn-Doped TiO2Nanotubes Fabricated by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2018, 10, 33287–33295. [Google Scholar] [CrossRef]
- Nikkanen, J. Synthesis of TiO2 by Various Methods Structural Characteristics, Photocatalytic Activity and Usability of Powders and Coatings; Tampere University of Technology: Tampere, Finland, 2016; ISBN 9789521538407. [Google Scholar]
- Foura, G.; Chouchou, N.; Soualah, A.; Kouachi, K.; Guidotti, M.; Robert, D. Fe-Doped TiO2 Supported on HY Zeolite for Solar Photocatalytic Treatment of Dye Pollutants. Catalysts 2017, 7, 344. [Google Scholar] [CrossRef]
- Cihlar, J.; Cihlar, J.; Bartonickova, E. Low-Temperature Sol-Gel Synthesis of Anatase Nanoparticles Modified by Au, Pd and Pt and Activity of TiO2/Au, Pd, Pt Photocatalysts in Water Splitting. J. Sol-Gel Sci. Technol. 2013, 65, 430–442. [Google Scholar] [CrossRef]
- Cihlar, J.; Kasparek, V.; Kralova, M.; Castkova, K. Biphasic Anatase-Brookite Nanoparticles Prepared by Sol-Gel Complex Synthesis and Their Photocatalytic Activity in Hydrogen Production. Int. J. Hydrogen Energy 2015, 40, 2950–2962. [Google Scholar] [CrossRef]
- Ceballos-Chuc, M.C.; Ramos-Castillo, C.M.; Alvarado-Gil, J.J.; Oskam, G.; Rodríguez-Gattorno, G. Influence of Brookite Impurities on the Raman Spectrum of TiO2 Anatase Nanocrystals. J. Phys. Chem. C 2018, 122, 19921–19930. [Google Scholar] [CrossRef]
- Yang, M.; Chen, P.; Tsai, M.; Chen, T.; Chang, I.; Chiu, H.; Lee, C. Anatase and Brookite TiO2 with Various Morphologies and Their Proposed Building Block. CrystEngComm 2014, 16, 441–447. [Google Scholar] [CrossRef]
- Drobná, H.; Dubnová, L.; Rokici, A. Nd/TiO2 Anatase-Brookite Photocatalysts for Photocatalytic Decomposition of Methanol. Front. Chem. 2018, 6, 44. [Google Scholar] [CrossRef]
- Li, J.; Ishigaki, T.; Sun, X. Anatase, Brookite, and Rutile Nanocrystals via Redox Reactions under Mild Hydrothermal Conditions: Phase-Selective Synthesis and Physicochemical Properties. J. Phys. Chem. C 2007, 111, 4969–4976. [Google Scholar] [CrossRef]
- Yan, Z.; Yin, K.; Xu, M.; Fang, N.; Yu, W.; Chu, Y.; Shu, S. Photocatalysis for Synergistic Water Remediation and H2 Production: A Review. Chem. Eng. J. 2023, 472, 145066. [Google Scholar] [CrossRef]
- Mandari, K.K.; Police, A.K.R.; Do, J.Y.; Kang, M.; Byon, C. Rare Earth Metal Gd Influenced Defect Sites in N Doped TiO2: Defect Mediated Improved Charge Transfer for Enhanced Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 2073–2082. [Google Scholar] [CrossRef]
- D’Elia, D.; Beauger, C.; Hochepied, J.F.; Rigacci, A.; Berger, M.H.; Keller, N.; Keller-Spitzer, V.; Suzuki, Y.; Valmalette, J.C.; Benabdesselam, M.; et al. Impact of Three Different TiO2 Morphologies on Hydrogen Evolution by Methanol Assisted Water Splitting: Nanoparticles, Nanotubes and Aerogels. Int. J. Hydrogen Energy 2011, 36, 14360–14373. [Google Scholar] [CrossRef]
- Yasuda, M.; Matsumoto, T.; Yamashita, T. Sacrificial Hydrogen Production over TiO2-Based Photocatalysts: Polyols, Carboxylic Acids, and Saccharides. Renew. Sustain. Energy Rev. 2018, 81, 1627–1635. [Google Scholar] [CrossRef]
- Do, H.H.; Nguyen, D.L.T.; Nguyen, X.C.; Le, T.H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.V.N.; Kim, S.Y.; Le, Q. Van Recent Progress in TiO2-Based Photocatalysts for Hydrogen Evolution Reaction: A Review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Afzal, M.J.; Pervaiz, E.; Farrukh, S.; Ahmed, T.; Bingxue, Z.; Yang, M. Highly Integrated Nanocomposites of RGO/TiO2nanotubes for Enhanced Removal of Microbes from Water. Environ. Technol. 2018, 40, 2567–2576. [Google Scholar] [CrossRef]
- Cihlar, J.; Navarro, L.K.T.; Cihlar, J.; Kasparek, V.; Michalicka, J.; Castkova, K.; Lazar, I.; Kastyl, J.; Celko, L.; Vesely, M.; et al. Influence of Substituted Acetic Acids on “Bridge” Synthesis of Highly Photocatalytic Active Heterophase TiO2 in Hydrogen Production. J. Sol-Gel Sci. Technol. 2022, 105, 471–488. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Toxqui-Teran, A.; Vega-Becerra, O.; Miki-Yoshida, M.; Rojas-Villalobos, M.; García-Guaderrama, M.; Aguilar-Martínez, J.A. Low-Temperature Synthesis and Characterization of Anatase TiO2 Nanoparticles by an Acid Assisted Sol-Gel Method. J. Alloys Compd. 2015, 647, 627–636. [Google Scholar] [CrossRef]
- Haggerty, J.E.S.; Schelhas, L.T.; Kitchaev, D.A.; Mangum, J.S.; Garten, L.M.; Sun, W.; Stone, K.H.; Perkins, J.D.; Toney, M.F.; Ceder, G.; et al. High-Fraction Brookite Films from Amorphous Precursors. Sci. Rep. 2017, 7, 15232. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Fornasiero, P. Brookite: Nothing New under the Sun? Catalysts 2017, 7, 304. [Google Scholar] [CrossRef]
- Bhave, R. Synthesis and Photocatalysis Study of Brookite Phase Titanium Dioxide Nanoparticles. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2007; pp. 1–73. [Google Scholar]
- Ma, L.; Zhang, T.; Song, R.; Guo, L. In-Situ Raman Study of Relation between Microstructure and Photoactivity of CdS@TiO2 Core-Shell Nanostructures. Int. J. Hydrogen Energy 2018, 43, 13778–13787. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Cao, Y.; Xiang, J.; Gao, H. Enhanced Photocatalytic Activities of Low-Bandgap TiO2-Reduced Graphene Oxide Nanocomposites. J. Nanoparticle Res. 2017, 19, 200. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A Review on TiO2-Based Nanotubes Synthesized via Hydrothermal Method: Formation Mechanism, Structure Modification, and Photocatalytic Applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Trapalis, C. Effects of Calcination Temperature on the Microstructures and Photocatalytic Activity of Titanate Nanotubes. J. Mol. Catal. A Chem. 2006, 249, 135–142. [Google Scholar] [CrossRef]
- Jbeli, A.; Ferraria, A.M.; Botelho do Rego, A.M.; Boufi, S.; Bouattour, S. Hybrid Chitosan-TiO2/ZnS Prepared under Mild Conditions with Visible-Light Driven Photocatalytic Activity. Int. J. Biol. Macromol. 2018, 116, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, G.; Zhou, Z.; Jiang, Y.; Hu, Q.; Xue, C.; Guo, W. Efficient Photocatalytic Hydrogen Production over Rh and Nb Codoped TiO2 Nanorods. Chem. Eng. J. 2018, 337, 282–289. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Jiao, S.; Wang, X.; Li, L.; Fang, Z.; Pang, G.; Feng, S. Synthesis of Blue Anatase TiO2 Nanoplates with {001} Facets and in Situ Noble Metal Anchoring. Dye. Pigment. 2016, 129, 191–198. [Google Scholar] [CrossRef]
- Moussaoui, R.; Elghniji, K.; ben Mosbah, M.; Elaloui, E.; Moussaoui, Y. Sol–Gel Synthesis of Highly TiO2 Aerogel Photocatalyst via High Temperature Supercritical Drying. J. Saudi Chem. Soc. 2017, 21, 751–760. [Google Scholar] [CrossRef]
- Habibi, S.; Jamshidi, M. Synthesis of TiO2 Nanoparticles Coated on Cellulose Nanofibers with Different Morphologies: Effect of the Template and Sol-Gel Parameters. Mater. Sci. Semicond. Process. 2020, 109, 104927. [Google Scholar] [CrossRef]
- Khan, H.; Berk, D. Effect of a Chelating Agent on the Physicochemical Properties of TiO2: Characterization and Photocatalytic Activity. Catal. Lett. 2014, 144, 890–904. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-,N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A.; Grabowska, E.; Marchelek, M.; Paszkiewicz-Gawron, M.; Zaleska-Medynska, A. Metal Oxide Photocatalysts. Met. Oxide-Based Photocatal. 2018, 51–209. [Google Scholar] [CrossRef]
- Roy, D.; Yadav, A.K. Green TiO2–ZnO Nanocomposite Stimulator for the Growth of Solanum Lycopersicum in Aquaculture. Appl. Nanosci. 2022, 12, 1403–1423. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO Nanoparticles Immobilized on Clay in Wastewater Treatment: A Review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef]
- Chen, Y.W.; Hsu, Y.H. Effects of Reaction Temperature on the Photocatalytic Activity of TiO2 with Pd and Cu Cocatalysts. Catalysts 2021, 11, 966. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, X.; Wang, L.; Wu, F.; Liu, S.; Chang, C.; Luo, X. A Simple Strategy to Design 3-Layered Au-TiO2 Dual Nanoparticles Immobilized Cellulose Membranes with Enhanced Photocatalytic Activity. Carbohydr. Polym. 2020, 231, 115694. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Gao, F.; Mailhot, G.; Pan, G. Algae Decorated TiO2/Ag Hybrid Nanofiber Membrane with Enhanced Photocatalytic Activity for Cr(VI) Removal under Visible Light. Chem. Eng. J. 2017, 314, 622–630. [Google Scholar] [CrossRef]
- Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. [Google Scholar] [CrossRef]
- Houas, A.; Taha, K.; Hamadi, N.B. Preparation of novel X% Li/TiO2 (x = 1%, 2% and 4%) and their activities under visible light. Dig. J. Nanomater. Biostructures 2020, 15, 579–585. [Google Scholar] [CrossRef]
- Cihlar, J.; Tinoco Navarro, L.K.; Kasparek, V.; Michalicka, J.; Kastyl, J.; Castkova, K.; Celko, L. Influence of LA/Ti Molar Ratio on the Complex Synthesis of Anatase/Brookite Nanoparticles and Their Hydrogen Production. Int. J. Hydrogen Energy 2021, 46. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic Hydrogen Production Using Metal Doped TiO2: A Review of Recent Advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Sharma, S.D.; Saini, K.K.; Kant, C.; Sharma, C.P.; Jain, S.C. PhotoDegradation of Dye Pollutant under UV Light by Nano-Catalyst Doped Titania Thin Films. Appl. Catal. B Environ. 2008, 84, 233–240. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A Review of TiO2 Nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- El-Saeid, M.H.; Alotaibi, M.O.; Alshabanat, M.; Al-Anazy, M.M.; Alharbi, K.R.; Altowyan, A.S. Impact of Photolysis and TiO2 on Pesticides Degradation in Wastewater. Water 2021, 13, 655. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Venz, P.; Frost, R.; Bartlett, J.; Woolfrey, J.; Kloprogge, J. Thermal Transformations of Titania Hydrolysates Prepared from Tetraisopropoxytitanium(Iv). Thermochim. Acta 2000, 346, 73–82. [Google Scholar] [CrossRef]
- Isley, S.L.; Penn, R.L. Titanium Dioxide Nanoparticles: Effect of Sol-Gel PH on Phase Composition, Particle Size, and Particle Growth Mechanism. J. Phys. Chem. C 2008, 112, 4469–4474. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Feldhoff, A.; Robben, L.; Dillert, R.; Bahnemann, D.W. Tailored Titanium Dioxide Nanomaterials: Anatase Nanoparticles and Brookite Nanorods as Highly Active Photocatalysts. Chem. Mater. 2010, 22, 2050–2060. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A Review on H2 Production through Photocatalytic Reactions Using TiO2/TiO2-Assisted Catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Polymorphic Phase Transition among the Titania Crystal Structures Using a Solution-Based Approach: From Precursor Chemistry to Nucleation Process. Nanoscale 2014, 6, 11574–11632. [Google Scholar] [CrossRef]
- Košević, M.; Šekularac, G.; Živković, L.; Panić, V.; Nikolić, B. TiO2 From Colloidal Dispersion as Support in Pt/TiO2 Nanocomposite for Electrochemical Applications. Croat. Chem. Acta 2017, 90, 251–258. [Google Scholar] [CrossRef]
- Oh, Y.-C.; Li, X.; Cubbage, J.W.; Jenks, W.S. Mechanisms of Catalyst Action in the TiO2-Mediated Photocatalytic Degradation and Cis–Trans Isomerization of Maleic and Fumaric Acid. Appl. Catal. B Environ. 2004, 54, 105–114. [Google Scholar] [CrossRef]
- Leblebici, M.E.; Stefanidis, G.D.; Van Gerven, T. Comparison of Photocatalytic Space-Time Yields of 12 Reactor Designs for Wastewater Treatment. Chem. Eng. Process. Process Intensif. 2015, 97, 106–111. [Google Scholar] [CrossRef]
- Hao, R.; Jiang, B.; Li, M.; Xie, Y.; Fu, H. Fabrication of Mixed-Crystalline-Phase Spindle-like TiO2 for Enhanced Photocatalytic Hydrogen Production. Sci. China Mater. 2015, 58, 363–369. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Group, S.P.; Energia, D.; Informazione, I. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaim, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Moon, I.S. A Review on UV/TiO2 Photocatalytic Oxidation Process. Korean J. Chem. Eng. 2008, 25, 64–72. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review Doped-TiO2: A Review. Recent Pat. Eng. 2014, 2, 157–164. [Google Scholar] [CrossRef]
- Fan, C.; Xue, P.; Sun, Y. Preparation of Nano-TiO2 Doped with Cerium and Its Photocatalytic Activity. J. Rare Earths 2006, 24, 309–313. [Google Scholar] [CrossRef]
- Mahmoud, H.A.; Narasimharao, K.; Ali, T.T.; Khalil, K.M.S. Acidic Peptizing Agent Effect on Anatase-Rutile Ration and Photocatalytic Performance of TiO2 Nanoparticles. Nanoscale Res. Lett. 2018, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Arenas, L.T.; Simm, C.W.; Gushikem, Y.; Dias, S.L.P.; Moro, C.C.; Costa, T.M.H.; Benvenutti, E.V. Synthesis of Silica Xerogels with High Surface Area Using Acetic Acid as Catalyst. J. Braz. Chem. Soc. 2007, 18, 886–890. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Shi, Z.; Zhi, M.; Hong, Z. The Investigation of an Organic Acid Assisted Sol-Gel Method for Preparing Monolithic Zirconia Aerogels. RSC Adv. 2018, 8, 8011–8020. [Google Scholar] [CrossRef] [PubMed]
- Vorsina, I.; Grigorieva, T.; Barinova, A.; Lyakhov, N. Mechanochemical Interaction of Silicon Dioxide with Organic Acids. Ariel.Ac.Il 2011, 19, 236–247. [Google Scholar]
- Banerjee, B.; Amoli, V.; Maurya, A.; Sinha, A.K.; Bhaumik, A. Green Synthesis of Pt-Doped TiO2 Nanocrystals with Exposed (001) Facets and Mesoscopic Void Space for Photo-Splitting of Water under Solar Irradiation. Nanoscale 2015, 7, 10504–10512. [Google Scholar] [CrossRef]
- Patra, A.K.; Dutta, A.; Bhaumik, A. Synthesis of Cuboid-Shaped Single-Crystalline TiO2 Nanocrystals with High-Energy Facets {001} and Its Dye-Sensitized Solar Cell Application. J. Phys. Chem. C 2014, 118, 16703–16709. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; De Coss, R.; Oskam, G. Phase-Pure TiO2 Nanoparticles: Anatase, Brookite and Rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef]
- Polte, J. Fundamental Growth Principles of Colloidal Metal Nanoparticles—A New Perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef]
- Kulkarni, D.; Wachs, I.E. Isopropanol Oxidation by Pure Metal Oxide Catalysts: Number of Active Surface Sites and Turnover Frequencies. Appl. Catal. A Gen. 2002, 237, 121–137. [Google Scholar] [CrossRef]
- Campbell, L.K.; Na, B.K.; Ko, E.I. Synthesis and Characterization of Titania Aerogels. Chem. Mater. 1992, 4, 1329–1333. [Google Scholar] [CrossRef]
- Cho, H.-W.; Liao, K.-L.; Yang, J.-S.; Wu, J.-J. Revelation of Rutile Phase by Raman Scattering for Enhanced Photoelectrochemical Performance of Hydrothermally-Grown Anatase TiO2 Film. Appl. Surf. Sci. 2018, 440, 125–132. [Google Scholar] [CrossRef]
- Finnegan, M.P.; Zhang, H.; Banfield, J.F. Phase Stability and Transformation in Titania Nanoparticles in Aqueous Solutions Dominated by Surface Energy. J. Phys. Chem. C 2007, 111, 1962–1968. [Google Scholar] [CrossRef]
- Tseng, T.K.; Lin, Y.S.; Chen, Y.J.; Chu, H. A Review of Photocatalysts Prepared by Sol-Gel Method for VOCs Removal. Int. J. Mol. Sci. 2010, 11, 2336–2361. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Ma, J.; Zhao, Z.; Qi, L. Hydrothermal Preparation of Uniform Nanosize Rutile and Anatase Particles. Chem. Mater. 1995, 7, 663–671. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Deng, K.; Chen, X.; Zou, Z. Low Temperature Synthesis and Photocatalytic Activity of Rutile TiO2 Nanorod Superstructutes. J. Phys. Chem. C 2007, 111, 2709–2714. [Google Scholar] [CrossRef]
- Tinoco Navarro, L.K.; Cihlar, J.; Michalicka, J.; Kastyl, J.; Castkova, K. Effect of MCAA Synthesis and Calcination Temperature on Heterojunction Formation and Photocatalytic Activity of Biphasic TiO2 (B/A). Catal. Lett. 2023, 1, 1–21. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Castelo-Quibén, J.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Carbon–TiO2 Composites as High-Performance Supercapacitor Electrodes: Synergistic Effect between Carbon and Metal Oxide Phases. J. Mater. Chem. A 2018, 6, 633–644. [Google Scholar] [CrossRef]
- Fischer, K.; Gawel, A.; Rosen, D.; Krause, M.; Latif, A.A.; Griebel, J.; Prager, A.; Schulze, A. Low-Temperature Synthesis of Anatase/Rutile/Brookite TiO2 Nanoparticles on a Polymer Membrane for Photocatalysis. Catalysts 2017, 7, 209. [Google Scholar] [CrossRef]
- Cihlar, J.; Vrba, R.; Castkova, K.; Cihlar, J. Effect of Transition Metal on Stability and Activity of La-Ca-M-(Al)-O (M = Co, Cr, Fe and Mn) Perovskite Oxides during Partial Oxidation of Methane. Int. J. Hydrogen Energy 2017, 42, 19920–19934. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Shape and Aggregation Control of Nanoparticles: Not Shaken, Not Stirred. J. Am. Chem. Soc. 2006, 128, 968–975. [Google Scholar] [CrossRef]
- Jana, S. Advances in nanoscale alloys and intermetallics: Low temperature solution chemistry synthesis and application in catalysis. Dalton Trans. 2015, 44, 18692–18717. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D. Core-Shell Structured Titanium Dioxide Nanomaterials for Solar Energy Utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Moral, L.M.; Romero, A.; Holz, F.; Rueda, M.; Navarrete, A.; Martín, A. Tuned Pd/SiO2 Aerogel Catalyst Prepared by Different Synthesis Techniques. J. Taiwan Inst. Chem. Eng. 2016, 65, 515–521. [Google Scholar] [CrossRef]
- Justh, N.; Mikula, G.J.; Bakos, L.P.; Nagy, B.; László, K.; Parditka, B.; Erdélyi, Z.; Takáts, V.; Mizsei, J.; Szilágyi, I.M. Photocatalytic Properties of TiO2 @polymer and TiO2 @carbon Aerogel Composites Prepared by Atomic Layer Deposition. Carbon N. Y. 2019, 147, 476–482. [Google Scholar] [CrossRef]
- Linhares, T.; Pessoa De Amorim, M.T.; Durães, L. Silica Aerogel Composites with Embedded Fibres: A Review on Their Preparation, Properties and Applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Alwin, S.; Shajan, X.S. Aerogels: Promising Nanostructured Materials for Energy Conversion and Storage Applications. Mater. Renew. Sustain. Energy 2020, 4, 7. [Google Scholar] [CrossRef]
- Koparde, V.N.; Cummings, P.T. Phase Transformations during Sintering of Titania Nanoparticles. ACS Nano 2008, 2, 1620–1624. [Google Scholar] [CrossRef]
- RSC TiO2: Manufacture of Titanium Dioxide. R. Soc. Chem. 2016, 5.
- Mao, Q.; Ren, Y.; Luo, K.H.; Li, S. Sintering-Induced Phase Transformation of Nanoparticles: A Molecular Dynamics Study. J. Phys. Chem. C 2015, 119, 28631–28639. [Google Scholar] [CrossRef]
- Ren, L.; Cui, S.; Cao, F.; Guo, Q. An Easy Way to Prepare Monolithic Inorganic Oxide Aerogels. Angew. Chem. Int. Ed. 2014, 53, 10147–10149. [Google Scholar] [CrossRef]
- Puskelova, J.; Baia, L.; Vulpoi, A.; Baia, M.; Antoniadou, M.; Dracopoulos, V.; Stathatos, E.; Gabor, K.; Pap, Z.; Danciu, V.; et al. Photocatalytic Hydrogen Production Using TiO2-Pt Aerogels. Chem. Eng. J. 2014, 242, 96–101. [Google Scholar] [CrossRef]
- Woignier, T.; Phalippou, J.; Despetis, F.; Aerogel, S.C.; Lisa, P. Aerogel Processing. 2018. Available online: https://amu.hal.science/hal-01930044/document (accessed on 2 December 2023).
- Serpone, N.; Artemev, Y.M.; Ryabchuk, V.K.; Emeline, A.V.; Horikoshi, S. Light-Driven Advanced Oxidation Processes in the Disposal of Emerging Pharmaceutical Contaminants in Aqueous Media: A Brief Review. Curr. Opin. Green Sustain. Chem. 2017, 6, 18–33. [Google Scholar] [CrossRef]
- Reza Gholipour, M.; Dinh, C.T.; Béland, F.; Do, T.O. Nanocomposite Heterojunctions as Sunlight-Driven Photocatalysts for Hydrogen Production from Water Splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef] [PubMed]
- Dalod, A.R.M.; Henriksen, L.; Grande, T.; Einarsrud, M. Functionalized TiO2 Nanoparticles by Single-Step Hydrothermal Synthesis: The Role of the Silane Coupling Agents. Beilstein J. Nanotechnol. 2017, 8, 304–312. [Google Scholar] [CrossRef]
- Franceschini, E.A.; Gomez, M.J.; Lacconi, G.I. One Step Synthesis of High Efficiency Nickel/Mesoporous TiO2 Hybrid Catalyst for Hydrogen Evolution Reaction. J. Energy Chem. 2019, 29, 79–87. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile?—Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2015, 4, 4043. [Google Scholar] [CrossRef] [PubMed]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Moradi, V.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Significant Improvement in Visible Light Photocatalytic Activity of Fe Doped TiO2 Using an Acid Treatment Process. Appl. Surf. Sci. 2018, 427, 791–799. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Li, J.; Yang, X. Photocatalytic Hydrogen Production from Methanol Aqueous Solution under Visible-Light Using Cu/S–TiO2 Prepared by Electroless Plating Method. Catal. Commun. 2015, 59, 189–194. [Google Scholar] [CrossRef]
- Patel, R.; Patel, S. Renewable Hydrogen Production from Butanol: A Review. Clean Energy 2017, 1, 90–101. [Google Scholar] [CrossRef]
- Huang, L.; Li, R.; Chong, R.; Liu, G.; Han, J.; Li, C. Cl- Making Overall Water Splitting Possible on TiO2-Based Photocatalysts. Catal. Sci. Technol. 2014, 4, 2913–2918. [Google Scholar] [CrossRef]
- Hippargi, G.; Mangrulkar, P.; Chilkalwar, A.; Labhsetwar, N.; Rayalu, S. Chloride Ion: A Promising Hole Scavenger for Photocatalytic Hydrogen Generation. Int. J. Hydrogen Energy 2018, 43, 6815–6823. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Photocatalytic Performance of Aerogels for Organic Dyes Removal from Wastewaters: Review Study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Tinoco Navarro, L.K.; Bednarikova, V.; Kastyl, J.; Cihlar, J. Structure and Photocatalytic Properties of Ni-, Co-, Cu-, and Fe-Doped TiO2 Aerogels. Gels 2023, 9, 357. [Google Scholar] [CrossRef]
- Oh, W.; Yv, W.; Jung, C. Synthesis and Characterization of Fe-Containing C/TiO2 Composites and Their Degradation Effect for the Piggery Waste. Processing 2008, 13, 18–20. [Google Scholar]
- Lim, T.T.; Yap, P.S.; Srinivasan, M.; Fane, A.G. TiO2/AC Composites for Synergistic Adsorption-Photocatalysis Processes: Present Challenges and Further Developments for Water Treatment and Reclamation. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1173–1230. [Google Scholar] [CrossRef]
- Zhu, C.; Yao, H.; Sun, T.; Le, S.; Jin, Q.; Chen, C.; Xu, H.; Wang, S. Ultrathin Fluorine-Doped TiO2(B) Nanosheets-Anchored Hierarchical Cog Wheel-Shaped NH2-MIL-53(Al) for Boosting Photocatalytic Activity. Chem. Eng. J. 2023, 460, 141849. [Google Scholar] [CrossRef]
- Dozzi, M.V.; D’Andrea, C.; Ohtani, B.; Valentini, G.; Selli, E. Fluorine-Doped TiO2 Materials: Photocatalytic Activity vs. Time-Resolved Photoluminescence. J. Phys. Chem. C 2013, 117, 25586–25595. [Google Scholar] [CrossRef]
- Boningari, T.; Inturi, S.N.R.; Suidan, M.; Smirniotis, P.G. Novel One-Step Synthesis of Sulfur Doped-TiO2 by Flame Spray Pyrolysis for Visible Light Photocatalytic Degradation of Acetaldehyde. Chem. Eng. J. 2018, 339, 249–258. [Google Scholar] [CrossRef]
- Ganesh, I.; Kumar, P.P.; Gupta, A.K.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and Characterization of Fe-Doped TiO2 Powders for Solar Light Response and Photocatalytic Applications. Process. Appl. Ceram. 2012, 6, 21–36. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.; Chiarello, G.L.; Selli, E.; Bomboi, G.; Calloni, A.; Bussetti, G.; Albani, G.; Bestetti, M.; Franz, S. Ni-Doped Titanium Dioxide Films Obtained by Plasma Electrolytic Oxidation in Refrigerated Electrolytes. Surfaces 2020, 3, 168–181. [Google Scholar] [CrossRef]
- Mahy, J.G.; Cerfontaine, V.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Heinrichs, B.; Lambert, S.D. Highly Efficient Low-Temperature N-Doped TiO2 Catalysts for Visible Light Photocatalytic Applications. Materials 2018, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Hart, J.N.; Boensch, D.; Scott, J.; Ng, Y.H.; Amal, R. Hydrogen Evolution via Glycerol Photoreforming over Cu–Pt Nanoalloys on TiO2. Appl. Catal. A Gen. 2016, 518, 221–230. [Google Scholar] [CrossRef]
- Rahul, T.K.; Mohan, M.; Sandhyarani, N. Enhanced Solar Hydrogen Evolution over in Situ Gold-Platinum Bimetallic Nanoparticle-Loaded Ti3+ Self-Doped Titania Photocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 3049–3059. [Google Scholar] [CrossRef]
- Di Liberto, G.; Tosoni, S.; Pacchioni, G. Nitrogen Doping in Coexposed (001)-(101) Anatase TiO2 Surfaces: A DFT Study. Phys. Chem. Chem. Phys. 2019, 21, 21497–21505. [Google Scholar] [CrossRef]
- Rivero, M.J.; Iglesias, O.; Ribao, P.; Ortiz, I. Kinetic Performance of TiO2/Pt/Reduced Graphene Oxide Composites in the Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 101–109. [Google Scholar] [CrossRef]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Amato, L.S.; Maddalena, P. Charge Carrier Processes and Optical Properties in TiO2 and TiO2-Based Heterojunction Photocatalysts: A Review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Han, X.; Liu, B.; Jiang, J.; Liu, S.; Zhang, J.; Shi, H. Direct Z-Scheme TiO2-x/AgI Heterojunctions for Highly Efficient Photocatalytic Degradation of Organic Contaminants and Inactivation of Pathogens. Sep. Purif. Technol. 2021, 261, 118306. [Google Scholar] [CrossRef]
- Ahmadi, N.; Nemati, A.; Bagherzadeh, M. Synthesis and Properties of Ce-Doped TiO2-Reduced Graphene Oxide Nanocomposite. J. Alloys Compd. 2018, 742, 986–995. [Google Scholar] [CrossRef]
- Ho, C.C.; Kang, F.; Chang, G.M.; You, S.J.; Wang, Y.F. Application of Recycled Lanthanum-Doped TiO2 Immobilized on Commercial Air Filter for Visible-Light Photocatalytic Degradation of Acetone and NO. Appl. Surf. Sci. 2019, 465, 31–40. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Ram, C. Calcination Temperature Effect on Titanium Oxide (TiO2) Nanoparticles Synthesis. Opt. Stuttg. 2021, 241, 166934. [Google Scholar] [CrossRef]
- Jiang, D.; Otitoju, T.A.; Ouyang, Y.; Shoparwe, N.F.; Wang, S.; Zhang, A.; Li, S. A Review on Metal Ions Modified TiO2 for Photocatalytic Degradation of Organic Pollutants. Catalysts 2021, 11, 1039. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; León, R. Effect of Doping TiO2 NPs with Lanthanides (La, Ce and Eu) on the Adsorption and Photodegradation of Cyanide—A Comparative Study. Nanomaterials 2023, 13, 1068. [Google Scholar] [CrossRef] [PubMed]
- Keerthana, S.P.; Yuvakkumar, R.; Ravi, G.; Hong, S.I.; Al-Sehemi, A.G.; Velauthapillai, D. Fabrication of Ce Doped TiO2 for Efficient Organic Pollutants Removal from Wastewater. Chemosphere 2022, 293, 133540. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Wang, X.; Li, H.; Zhu, L.; Xu, X.; Jiang, S. Enhancing CO2 Photoconversion Activity of TiO2 via Synergistic Effects of La-Doping and Ammonia Vapor Heat Treatment. J. Photochem. Photobiol. A Chem. 2023, 441, 114755. [Google Scholar] [CrossRef]
- Dubey, R.S.; Jadkar, S.R.; Bhorde, A.B. Synthesis and Characterization of Various Doped TiO2 Nanocrystals for Dye-Sensitized Solar Cells. ACS Omega 2021, 6, 3470–3482. [Google Scholar] [CrossRef]
- Liu, J.; Hodes, G.; Yan, J.; Liu, S. (Frank) Metal-Doped Mo2C (Metal = Fe, Co, Ni, Cu) as Catalysts on TiO2 for Photocatalytic Hydrogen Evolution in Neutral Solution. Chin. J. Catal. 2020, 42, 205–216. [Google Scholar] [CrossRef]
- Vargas Hernández, J.; Coste, S.; García Murillo, A.; Carrillo Romo, F.; Kassiba, A. Effects of Metal Doping (Cu, Ag, Eu) on the Electronic and Optical Behavior of Nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Kim, M.G.; Kang, J.M.; Lee, J.E.; Kim, K.S.; Kim, K.H.; Cho, M.; Lee, S.G. Effects of Calcination Temperature on the Phase Composition, Photocatalytic Degradation, and Virucidal Activities of TiO2Nanoparticles. ACS Omega 2021, 6, 10668–10678. [Google Scholar] [CrossRef] [PubMed]
- Hamad, H.; Bailón-García, E.; Morales-Torres, S.; Pérez-Cadenas, A.F.; Carrasco-Marín, F.; Maldonado-Hódar, F.J. Cellulose–TiO2 Composites for the Removal of Water Pollutants. In Bio-Based Materials and Biotechnologies for Eco-Efficient Construction; Woodhead Publishing: Sawston, UK, 2020; pp. 329–358. [Google Scholar] [CrossRef]
- Xin, W.; Gao, T.; Zhang, W.; Hu, T.; Sun, X.; Zhou, G. Three-Dimensional Hollow SnO2@TiO2 Spheres Encapsulated in Reduced Graphene Oxide Aerogels as Promising Anodes for Lithium-Ion Storage. J. Alloys Compd. 2019, 784, 157–164. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Bakbolat, B.; Baimenov, A.; Issadykov, A.; Yeleuov, M.; Daulbayev, C. Photocatalysts for a Sustainable Future: Innovations in Large-Scale Environmental and Energy Applications. Sci. Total Environ. 2023, 885, 163914. [Google Scholar] [CrossRef] [PubMed]
- Gurushantha, K.; Kottam, N.; Smrithi, S.P.; Dharmaprakash, M.S.; Keshavamurthy, K.; Meena, S.; Srinatha, N. Visible Light Active WO3/TiO2 Heterojunction Nanomaterials for Electrochemical Sensor, Capacitance and Photocatalytic Application s. Catal. Lett. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- Turlybekuly, A.; Sarsembina, M.; Mentbayeva, A.; Bakenov, Z.; Soltabayev, B. CuO/TiO2 Heterostructure-Based Sensors for Conductometric NO2 and N2O Gas Detection at Room Temperature. Sens. Actuators B Chem. 2023, 397, 134635. [Google Scholar] [CrossRef]
- Tian, X.; Hu, Z.; Wang, T.; Wang, H.; Zhang, Q.; Wei, X. Influence of Multi-Layer TiO2/SnO2 Heterojunctions on Fast and Sensitive Ethanol Detection. Vacuum 2023, 207, 111620. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Liang, Q.; Zhang, J.; Liu, J.; Liu, Y.; Wang, C.; Gao, J.; Lu, H. Nanoporous Co3O4-TiO2Heterojunction Nanosheets for Ethanol Sensing. ACS Appl. Nano Mater. 2022, 5, 4779–4786. [Google Scholar] [CrossRef]
- Galstyan, V.; Poli, N.; Golovanov, V.; D’Arco, A.; Macis, S.; Lupi, S.; Bolli, E.; Kaciulis, S.; Mezzi, A.; Comini, E. Tunable Chemical Reactivity and Selectivity of WO3/TiO2 Heterojunction for Gas Sensing Application s. Adv. Mater. Technol. 2022, 7, 2201751. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Lee, S.Y.; Gwon, J.G.; Cho, H.J.; Wu, Q.; Kim, Y.H.; Lee, W.H. Photocatalytic Performance of Highly Transparent and Mesoporous Molybdenum-Doped Titania Films Fabricated by Templating Cellulose Nanocrystals. Ceram. Int. 2018, 44, 16647–16653. [Google Scholar] [CrossRef]
- Bakre, P.V.; Tilve, S.G. Direct Access to Highly Crystalline Mesoporous Nano TiO2 Using Sterically Bulky Organic Acid Templates. J. Phys. Chem. Solids 2018, 116, 234–240. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Skirtach, A.G.; Peretiatko, T.; Budkowski, A. Passive Antifouling and Active Self-Disinfecting Antiviral Surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef]
- Zhu, H.; Tan, J.; Qiu, J.; Wang, D.; Zhao, Z.; Lu, Z.; Huang, G.; Liu, X.; Mei, Y. Gold Nanoparticles Decorated Titanium Oxide Nanotubes with Enhanced Antibacterial Activity Driven by Photocatalytic Memory Effect. Coatings 2022, 12, 1351. [Google Scholar] [CrossRef]
- Tomás-Gamasa, M.; Mascareñas, J.L. TiO2-Based Photocatalysis at the Interface with Biology and Biomedicine. ChemBioChem 2020, 21, 294–309. [Google Scholar] [CrossRef]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 Nanocomposite Coatings for Surfaces, Dental and Orthopaedic Implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef]
- Wang, R.; Shi, M.; Xu, F.; Qiu, Y.; Zhang, P.; Shen, K.; Zhao, Q.; Yu, J.; Zhang, Y. Graphdiyne-Modified TiO2 Nanofibers with Osteoinductive and Enhanced Photocatalytic Antibacterial Activities to Prevent Implant Infection. Nat. Commun. 2020, 11, 4465. [Google Scholar] [CrossRef]
- Song, P.; Wang, M.L.; Duan, Y.X.; Wang, A.J.; Xue, Y.; Mei, L.P.; Feng, J.J. Bifunctional Photoelectrochemical Aptasensor Based on Heterostructured Ag3PO4/Ag/TiO2 Nanorod Array for Determination of Two Tumor Markers. Microchim. Acta 2023, 190, 85. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Myung, J. ha Highly Selective Detection of Acetone by TiO2-SnO2 Heterostructures for Environmental Biomarkers of Diabetes. Sens. Actuators B Chem. 2021, 349, 130733. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rajabzadeh-Khosroshahi, M.; Eshaghi, M.M.; Rahmani, E.; Motasadizadeh, H.; Arshad, R.; Rahdar, A.; Pandey, S. TiO2-Based Nanocomposites for Cancer Diagnosis and Therapy: A Comprehensive Review. J. Drug Deliv. Sci. Technol. 2023, 82, 104370. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Y.; Wang, H.; Yang, D.; Liu, C.; Dou, W.; Jiang, X.; Deng, H.; Yang, R. Regulation of TiO2 @PVDF Piezoelectric Nanofiber Membranes on Osteogenic Differentiation of Mesenchymal Stem Cells. Nano Energy 2023, 115, 108742. [Google Scholar] [CrossRef]
- Ramesh, S.; Govarthanan, K.; Palaniappan, A. TiO2 Nanostructures—A Double Edged Sword: Current Progress on Their Role in Stem Cells’ Differentiation, Cancer Therapy, and Their Toxicity Issues. Nanotoxicology 2023, 17, 176–201. [Google Scholar] [CrossRef] [PubMed]








| Doping Methods | Advantages | Limitations |
|---|---|---|
| No Metallic Doping (C, N, S, F, Cl) [34,35,118,121] |
|
|
| Metallic Doping Methods | ||
| Transition Metal Doping (Fe, Co, Ni, Cu) [41,103,109,117] |
|
|
| Nobel Metal Doping (Pt, Au, Pd, Ag, Rd, Os) [19,38,122,123,124] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinoco Navarro, L.K.; Jaroslav, C. Enhancing Photocatalytic Properties of TiO2 Photocatalyst and Heterojunctions: A Comprehensive Review of the Impact of Biphasic Systems in Aerogels and Xerogels Synthesis, Methods, and Mechanisms for Environmental Applications. Gels 2023, 9, 976. https://doi.org/10.3390/gels9120976
Tinoco Navarro LK, Jaroslav C. Enhancing Photocatalytic Properties of TiO2 Photocatalyst and Heterojunctions: A Comprehensive Review of the Impact of Biphasic Systems in Aerogels and Xerogels Synthesis, Methods, and Mechanisms for Environmental Applications. Gels. 2023; 9(12):976. https://doi.org/10.3390/gels9120976
Chicago/Turabian StyleTinoco Navarro, Lizeth Katherine, and Cihlar Jaroslav. 2023. "Enhancing Photocatalytic Properties of TiO2 Photocatalyst and Heterojunctions: A Comprehensive Review of the Impact of Biphasic Systems in Aerogels and Xerogels Synthesis, Methods, and Mechanisms for Environmental Applications" Gels 9, no. 12: 976. https://doi.org/10.3390/gels9120976