Allelopathic Potential and Active Substances from Wedelia Chinensis (Osbeck)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Collection
2.2. Test Plant Species
2.3. Extraction
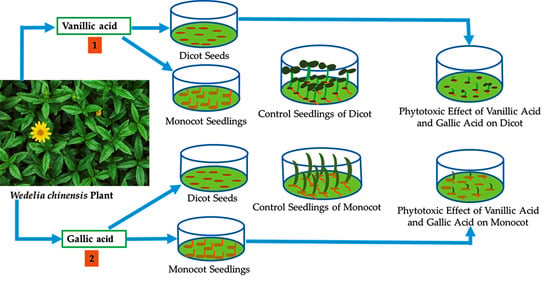
2.4. Growth Bioassay
2.5. Extract Partitions
2.6. Isolation and Purification of the Active Substances
2.7. Bioassay of the Isolated Substances
2.8. Statistics
3. Results
3.1. Allelopathic Effects of Wedelia chinensis on the Seedling Growth of the Tested Plant Species
3.2. Identification of the Phytotoxic Substances
3.3. Biological Activity of the Isolated Substances
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Santos, P.C.; Santos, V.H.M.; Mecina, G.F.; Andrade, A.R.; Fegueiredo, P.A.; Moraes, V.M.O.; Silva, L.P.; Silva, R.M.G. Phytotoxicity of Tegetes erecta L. and Tegetes patula L. on plant germination and growth. S. Afr. J. Bot. 2015, 100, 114–121. [Google Scholar]
- Crowder, D.W.; Reganold, J.P. Financial competitiveness of organic agriculture on a global scale. Proc. Natl. Acad. Sci. USA 2015, 112, 7611–7616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment. In Plant, Soil and Microbes; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 253–269. [Google Scholar]
- Chai, M.; Zhu, X.; Cui, H.; Jiang, C.; Zhang, J.; Shi, L. Lily Cultivars Have Allelopathic Potential in Controlling Orobanche aegyptiaca Persoon. PLoS ONE 2015, 10, e0142811. [Google Scholar] [CrossRef] [Green Version]
- Das, K.R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic potential and identification of phytotoxic substances in Cassia alata Linn. leaves. Acta Agric. Scand. Sect. B Plant Soil Sci. 2019, 69, 1–10. [Google Scholar] [CrossRef]
- Suwitchayanon, P.; Suenaga, K.; Iwasaki, A.; Kato-Noguchi, H. Myrislignan, a Growth Inhibitor from the Roots of Citronella grass. Nat. Prod. Commun. 2017, 12, 1077–1078. [Google Scholar] [CrossRef] [Green Version]
- Gomaa, N.H.; AbdElgawad, H.R. Phytotoxic effects of Echinochloa colona (L.) Link. (Poaceae) extracts on the germination and seedling growth of weeds. Span. J. Agric. Res. 2012, 10, 492. [Google Scholar] [CrossRef] [Green Version]
- Asaduzzaman, M.; An, M.; Pratley, J.E.; Luckett, D.J.; Lemerle, D. Canola (Brassica napus) germplasm shows variable allelopathic effects against annual ryegrass (Lolium rigidum). Plant Soil 2014, 380, 47–56. [Google Scholar] [CrossRef]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C.; Asolkar, L.V.; Kakkar, K.K.; Chakre, O.J.; Varma, B.S. Glossary of Indian Medicinal Plants; Council of Scientific & Industrial Research: New Delhi, India, 1956; p. 258. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; Bishen Singh Mahendra Pal Singh: Dehradun, India, 2006; pp. 1364–1365. [Google Scholar]
- Sharma, A.; Anand, K.; Pushpangadan, P.; Chandan, B.; Chopra, C.; Prabhakar, Y.S.; Damodaran, N. Hepatoprotective effects of Wedelia calendulacea. J. Ethnopharmacol. 1989, 25, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, B.; Azam, F.M.S.; Khatun, M.A.; Rahman, F.; Rahmatullah, M. Simultaneous shoot regeneration and rhizogenesis of Wedelia chinensis for In Vitro clonal propagation. Am. Eurasian J. Sustain. Agric. 2010, 4, 65–69. [Google Scholar]
- Kumar, R.M.; Suresh, V.; Rajesh, S.V.; Kumar, N.S.; Arunachalam, G. Pharmacognostical studies of the plant Wedelia chinensis (Osbeck) MERR. Int. J. Pharm. Res. Dev. 2011, 2, 53–57. [Google Scholar]
- Meena, A.K.; Rao, M.M.; Meena, R.P.; Panda, P. Pharmacological and phytochemical evidences for the plants of Wedelia Genus–A review. Asian J. Pharm. Res. 2011, 1, 7–12. [Google Scholar]
- Umasankar, K.; Kumar, R.M.; Suresh, A.; Kumar, N.S.; Arunachalam, G.; Suresh, V. CNS activity of ethanol extract of Wedelia chinensis in experimental animals. Int. J. Pharm. Sci. Nanotechnol. 2010, 3, 881–886. [Google Scholar]
- Nomani, I.; Mazumder, A.; Chakrabarthy, G.S. Wedelia chinensis (Asteraceae)—An overview of a potent medicinal herb. Int. J. Pharm. Tech. Res. 2013, 5, 957–964. [Google Scholar]
- Mathew, K.M. Flora of Tamilnadu-Carnatic; The Rapinat Herbarium—St. Joseph’s College: Trichirapalli, India, 1983; Volume 2, p. 392. [Google Scholar]
- Li, X.; Wang, Y.-F.; Shi, Q.; Sauriol, F. A New 30-Noroleanane Saponin from Wedelia chinensis. Helvetica Chim. Acta 2012, 95, 1395–1400. [Google Scholar] [CrossRef]
- Qiu, Q.; Wu, X.; Li, G.Q.; Li, Y.L.; Wang, G.C. Chemical constituents from Wedelia chinensis. Chin. Tradit. Pat. Med. 2014, 36, 1000–1004. [Google Scholar]
- Manjamalai, A.; Grace, V.B. Antioxidant Activity of Essential Oils from Wedelia chinensis (Osbeck) In Vitro and In Vivo Lung Cancer Bearing C57BL/6 Mice. Asian Pac. J. Cancer Prev. 2012, 13, 3065–3071. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, T.; Talukdar, D. Response of antioxidative enzymes to arsenic-induced phytotoxicity in leaves of a medicinal daisy, Wedelia chinensis Merrill. J. Nat. Sci. Biol. Med. 2013, 4, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Manjamalai, A.; Jiflin, G.J.; Grace, V.M.B. Study on the effect of essential oil of Wedelia chinensis (Osbeck) against microbes and inflammation. Asian J. Pharm. Clin. Res. 2012, 5, 155–163. [Google Scholar]
- Tsai, C.-H.; Tzeng, S.-F.; Hsieh, S.C.; Lin, C.-Y.; Tsai, C.-J.; Chen, Y.-R.; Yang, Y.-C.; Chou, Y.-W.; Lee, M.-T.; Hsiao, P.-W. Development of a standardized and effect-optimized herbal extract of Wedelia chinensis for prostate cancer. Phytomedicine 2015, 22, 406–414. [Google Scholar] [CrossRef]
- Rensen, Z.; Xianglian, L.; Shiming, L.; Qiang, Z.; Huifen, T. Allelopathic potential of Wedelia chinensis and its allelochemicals. Acta Ecol. Sinica 1996, 16, 20–27. [Google Scholar]
- Chang, S.W.; Kim, K.H.; Lee, I.K.; Choi, S.U.; Ryu, S.Y.; Lee, K.R. Phytochemical Constituents of Bistorta manshuriensis. Nat. Prod. Sci. 2009, 15, 234–240. [Google Scholar]
- Al-Majmaie, S.; Nahar, L.; Sharples, G.P.; Wadi, K.; Sarker, S.D. Isolation and Antimicrobial Activity of Rutin and Its Derivatives from Ruta chalepensis (Rutaceae) Growing in Iraq. Rec. Nat. Prod. 2018, 13, 64–70. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Suzuki, M.; Noguchi, K.; Ohno, O.; Suenaga, K.; Laosinwattana, C. A Potent Phytotoxic Substance in Aglaia odorata Lour. Chem. Biodivers. 2016, 13, 549–554. [Google Scholar] [CrossRef]
- Islam, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of two potential phytotoxic substances from the aquatic fern Marsilea crenata. J. Plant Biol. 2017, 60, 75–81. [Google Scholar] [CrossRef]
- Appiah, K.S.; Mardani, H.K.; Omari, R.A.; Eziah, V.Y.; Ofosu-Anim, J.; Onwona-Agyeman, S.; Amoatey, C.A.; Kawada, K.; Katsura, K.; Oikawa, Y.; et al. Involvement of Carnosic Acid in the Phytotoxicity of Rosmarinus officinalis Leaves. Toxins 2018, 10, 498. [Google Scholar] [CrossRef] [Green Version]
- Boonmee, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic activity of leaf and stem extracts and identification of a phytotoxic substance from Caesalpinia mimosoides Lamk. Theor. Exp. Plant Physiol. 2018, 30, 129–139. [Google Scholar] [CrossRef]
- Islam, M.S.; Zaman, F.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic potential of Chrysopogon aciculatus (Retz.) Trin. (Poaceae). Weed Biol. Manag. 2019, 19, 51–58. [Google Scholar] [CrossRef]
- Rob, M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Activity and Identification of Phytotoxic Substances from Schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Đorđević, T.; Sarić-Krsmanović, M.; Umiljendić, J.G. Phenolic Compounds and Allelopathic Potential of Fermented and Unfermented Wheat and Corn Straw Extracts. Chem. Biodivers. 2019, 16, e1800420. [Google Scholar] [CrossRef]
- Chon, S.-U.; Jang, H.-G.; Kim, D.-K.; Kim, Y.-M.; Boo, H.-O. Allelopathic potential in lettuce (Lactuca sativa L.) plants. Sci. Hortic. 2005, 106, 309–317. [Google Scholar] [CrossRef]
- Benković, V.; Orsolić, N.; Knezević, A.H.; Ramić, S.; Dikić, D.; Basić, I.; Kopjar, N. Evaluation of the radioprotective effects of propolis and flavonoids in gamma-irradiated mice: The alkaline comet assay study. Biol. Pharm. Bull. 2008, 31, 167–172. [Google Scholar] [CrossRef] [Green Version]
- ArrayExpress—A Database of Functional Genomics Experiments. Available online: http://www.ebi.ac.uk/arrayexpress/ (accessed on 12 November 2012).
- European Medicinal Agency. Assessment Report on Angelica sinensis (Oliv.) Diels, Radix; EMA/HMPC/614586/2012; European Medicinal Agency: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Siriamornpun, S.; Kaewseejan, N. Quality, bioactive compounds and antioxidant capacity of selected climacteric fruits with relation to their maturity. Sci. Hortic. 2017, 221, 33–42. [Google Scholar] [CrossRef]
- Bevilacqua, A.; D’Amato, D.; Sinigaglia, M.; Corbo, M.R. Combination of Homogenization, Citrus Extract and Vanillic Acid for the Inhibition of Some Spoiling and Pathogenic Bacteria Representative of Dairy Microflora. Food Bioprocess Technol. 2012, 6, 2048–2058. [Google Scholar] [CrossRef]
- Kim, J.; Soh, S.Y.; Bae, H.; Nam, S. Antioxidant and phenolic contents in potatoes (Solanum tuberosum L.) and micropropagated potatoes. Appl. Biol. Chem. 2019, 62, 17. [Google Scholar] [CrossRef]
- Espinosa, R.R.; Inchingolo, R.; Alencar, S.M.; Rodriguez-Estrada, M.T.; Castro, I.A. Antioxidant activity of phenolic compounds added to a functional emulsion containing omega-3 fatty acids and plant sterol esters. Food Chem. 2015, 182, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical Composition, Antioxidant Properties, and Thermal Stability of a Phytochemical Enriched Oil from Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, L.; Moore, J.; Wu, T.; Wang, Z. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chem. 2009, 113, 160–165. [Google Scholar] [CrossRef]
- Kim, M.-C.; Kim, S.-J.; Kim, D.-S.; Jeon, Y.-D.; Park, S.J.; Lee, H.S.; Um, J.-Y.; Hong, S.-H. Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef]
- Zhao, C.; Jia, Y.; Lu, F. Angelica Stem: A Potential Low-Cost Source of Bioactive Phthalides and Phytosterols. Molecules 2018, 23, 3065. [Google Scholar] [CrossRef] [Green Version]
- Batish, D.R.; Lavanya, K.; Singh, H.P.; Kohli, R.K. Root-mediated Allelopathic Interference of Nettle-leaved Goosefoot (Chenopodium murale) on Wheat (Triticum aestivum). J. Agron. Crop. Sci. 2007, 193, 37–44. [Google Scholar] [CrossRef]
- Mitić, M.; Janković, S.; Mašković, P.; Arsić, B.; Mitić, J.; Ickovski, J. Kinetic models of the extraction of vanillic acid from pumpkin seeds. Open Chem. 2020, 18, 22–30. [Google Scholar] [CrossRef]
- Macías, F.A.; Simonet, A.M.; Galindo, J.C.G.; Castellano, D. Bioactive phenolics and polar compounds from Melilotus messanensis. Phytochemistry 1999, 50, 35–46. [Google Scholar] [CrossRef]
- Parveen, I.; Winters, A.; Threadgill, M.D.; Hauck, B.; Morris, P.; Shah, I.P. Extraction, structural characterisation and evaluation of hydroxycinnamate esters of orchard grass (Dactylis glomerata) as substrates for polyphenol oxidase. Phytochemistry 2008, 69, 2799–2806. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.V.; Cavalcante, F.; Vicentini, V. Different responses of vanillic acid, a phenolic compound, in HTC cells: Cytotoxicity, antiproliferative activity, and protection from DNA-induced damage. Genet. Mol. Res. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Sethupathy, S.; Ananthi, S.; Selvaraj, A.; Shanmuganathan, B.; Vigneshwari, L.; Balamurugan, K.; Mahalingam, S.; Pandian, S.K. Vanillic acid from Actinidia deliciosa impedes virulence in Serratia marcescens by affecting S-layer, flagellin and fatty acid biosynthesis proteins. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yemiş, G.P.; Pagotto, F.; Bach, S.; Delaquis, P. Effect of Vanillin, Ethyl Vanillin, and Vanillic Acid on the Growth and Heat Resistance of Cronobacter Species. J. Food Prot. 2011, 74, 2062–2069. [Google Scholar] [CrossRef]
- Yemiş, G.P.; Pagotto, F.; Bach, S.; Delaquis, P. Thermal Tolerance and Survival of Cronobacter sakazakiiin Powdered Infant Formula Supplemented with Vanillin, Ethyl Vanillin, and Vanillic Acid. J. Food Sci. 2012, 77, M523–M527. [Google Scholar] [CrossRef] [PubMed]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Huang, S.-M.; Hsu, C.-L.; Chuang, H.-C.; Shih, P.-H.; Wu, C.-H.; Yen, G.-C. Inhibitory effect of vanillic acid on methylglyoxal-mediated glycation in apoptotic Neuro-2A cells. NeuroToxicology 2008, 29, 1016–1022. [Google Scholar] [CrossRef]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective Effect of Syringic Acid and Vanillic Acid on Concanavalin A-Induced Liver Injury. Biol. Pharm. Bull. 2009, 32, 1215–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-J.; Kim, M.-C.; Um, J.-Y.; Hong, S.-H. The Beneficial Effect of Vanillic Acid on Ulcerative Colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Phytochemical Constituents of Gras Herbs and Other Economic Plants; CRC Press: Boca Raton, FL, USA, 1992; pp. 254–255. [Google Scholar]
- Kalinova, J.; Vrchotová, N.; Tříska, J. Exudation of Allelopathic Substances in Buckwheat (Fagopyrum esculentum Moench). J. Agric. Food Chem. 2007, 55, 6453–6459. [Google Scholar] [CrossRef]
- Shankar, S.R.M.; Girish, R.; Karthik, N.; Rajendran, R.; Mahendran, V.S. Allelopathic effects of phenolics and terpenoids extracted from Gmelina arborea on germination of black gram (Vigna mungo) and green gram (Vigna radiata). Allelopath. J. 2009, 23, 323–331. [Google Scholar]
- Zhang, T.-T.; Zheng, C.-Y.; Hu, W.; Xu, W.-W.; Wang, H.-F. The allelopathy and allelopathic mechanism of phenolic acids on toxic Microcystis aeruginosa. Environ. Boil. Fishes 2009, 22, 71–77. [Google Scholar] [CrossRef]
- Ghareib, H.R.A.; Abdelhamed, M.S.; Ibrahim, O.H. Antioxidative effects of the acetone fraction and vanillic acid from Chenopodium muraleon tomato plants. Weed Biol. Manag. 2010, 10, 64–72. [Google Scholar] [CrossRef]
- Khang, D.T.; Anh, L.H.; Ha, P.T.T.; Tuyen, P.T.; Van Quan, N.; Minh, L.T.; Quan, N.T.; Minh, T.N.; Xuan, T.D.; Khanh, T.D.; et al. Allelopathic Activity of Dehulled Rice and its Allelochemicals on Weed Germination. Int. Lett. Nat. Sci. 2016, 58, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kawada, M.; Ohno, Y.; Ri, Y.; Ikoma, T.; Yuugetu, H.; Asai, T.; Watanabe, M.; Yasuda, N.; Akao, S.; Takemura, G.; et al. Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice. Anti Cancer Drugs 2001, 12, 847–852. [Google Scholar] [CrossRef]
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Medicinal importance of gallic acid and its ester derivatives: A patent review. Pharm. Pat. Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandurangan, A.K.; Mohebali, N.; Norhaizan, M.E.; Looi, C.Y. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des. Dev. Ther. 2015, 9, 3923–3934. [Google Scholar] [CrossRef] [Green Version]
- Zucca, P.; Rosa, A.; Tuberoso, C.I.G.; Piras, A.; Rinaldi, A.; Sanjust, E.; Dessì, M.A.; Rescigno, A.; Sanjust, E. Evaluation of Antioxidant Potential of “Maltese Mushroom” (Cynomorium coccineum) by Means of Multiple Chemical and Biological Assays. Nutrients 2013, 5, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Carver, J.A.; Calabrese, A.N.; Pukala, T.L. Gallic acid interacts with α-synuclein to prevent the structural collapse necessary for its aggregation. Biochim. Biophys. Acta Proteins Proteom. 2014, 1844, 1481–1485. [Google Scholar] [CrossRef]
- Souza, M.C.; Santos, M.P.; Sumere, B.R.; Silva, L.C.; Cunha, D.T.; Martínez, J.; Barbero, G.F.; Rostagno, M.A. Isolation of gallic acid, caffeine and flavonols from black tea by on-line coupling of pressurized liquid extraction with an adsorbent for the production of functional bakery products. LWT 2020, 117, 108661. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Antimicrobial gallic acid from Caesalpinia mimosoides Lamk. Food Chem. 2007, 100, 1044–1048. [Google Scholar] [CrossRef]
- Mansouri, S.M.T.; Farbood, Y.; Sameri, M.J.; Sarkaki, A.; NaghiZadeh, B.; Rafeirad, M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2013, 138, 1028–1033. [Google Scholar] [CrossRef]
- Rafiee, S.A.; Farhoosh, R.; Sharif, A. Antioxidant Activity of Gallic Acid as Affected by an Extra Carboxyl Group than Pyrogallol in Various Oxidative Environments. Eur. J. Lipid Sci. Technol. 2018, 120. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Zhou, X.; Zhang, M.; Chen, Y.; Nie, S.; Xie, M. Combined application of gallate ester and α-tocopherol in oil-in-water emulsion: Their distribution and antioxidant efficiency. J. Dispers. Sci. Technol. 2019, 41, 909–917. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, X.; Zhang, K.; Zhu, L.; Zhou, F. Investigation of Gallic Acid Induced Anticancer Effect in Human Breast Carcinoma MCF-7 Cells. J. Biochem. Mol. Toxicol. 2014, 28, 387–393. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jun, C.-D.; Suk, K.; Choi, B.-J.; Lim, H.; Park, S.; Lee, S.H.; Shin, H.-Y.; Kim, D.-K.; Shin, T.-Y. Gallic Acid Inhibits Histamine Release and Pro-inflammatory Cytokine Production in Mast Cells. Toxicol. Sci. 2005, 91, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Asokkumar, K.; Umamaheswari, M.; Sivashanmugam, A.T.; SubhadraDevi, V. Antiulcerogenic Effect of Gallic Acid in Rats and its Effect on Oxidant and Antioxidant Parameters in Stomach Tissue. Indian J. Pharm. Sci. 2013, 75, 149–155. [Google Scholar] [PubMed]
- Akbari, G. Molecular mechanisms underlying gallic acid effects against cardiovascular diseases: An update review. Avicenna J. Phytomed. 2020, 10, 11–23. [Google Scholar]
- Chao, J.; Huo, T.-I.; Cheng, H.-Y.; Tsai, J.-C.; Liao, J.-W.; Lee, M.-S.; Qin, X.-M.; Hsieh, M.-T.; Pao, L.-H.; Peng, W.-H. Gallic Acid Ameliorated Impaired Glucose and Lipid Homeostasis in High Fat Diet-Induced NAFLD Mice. PLoS ONE 2014, 9, e96969. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.-W.; Chang, W.-C.; Wu, J.S.-B.; Shih, R.-W.; Shen, S.-C. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr. Res. 2016, 36, 150–160. [Google Scholar] [CrossRef]
- Kee, H.J.; Cho, S.-N.; Kim, G.R.; Choi, S.Y.; Ryu, Y.; Kim, I.K.; Hong, Y.J.; Park, H.W.; Ahn, Y.; Cho, J.G.; et al. Gallic acid inhibits vascular calcification through the blockade of BMP2–Smad1/5/8 signaling pathway. Vasc. Pharmacol. 2014, 63, 71–78. [Google Scholar] [CrossRef]
- Jin, L.; Lin, M.Q.; Piao, Z.H.; Cho, J.Y.; Kim, G.R.; Choi, S.Y.; Ryu, Y.; Sun, S.; Kee, H.J.; Jeong, M.H. Gallic acid attenuates hypertension, cardiac remodeling, and fibrosis in mice with NG-nitro-L-arginine methyl ester-induced hypertension via regulation of histone deacetylase 1 or histone deacetylase 2. J. Hypertens. 2017, 35, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Aslam, S.; Mehmood, T.; Naseer, R.; Nawaz, S.; Mujahid, H.; Firyal, S.; Anjum, A.A.; Sultan, A. Production of Gallic Acid Under Solid-State Fermentation by Utilizing Waste from Food Processing Industries. Waste Biomass Valoris. 2020, 1–9. [Google Scholar] [CrossRef]
- Dziga, D.; Suda, M.; Bialczyk, J.; Lechowski, Z.; Czaja-Prokop, U. The alteration of Microcystis aeruginosa biomass and dissolved microcystin-LR concentration following exposure to plant-producing phenols. Environ. Toxicol. 2007, 22, 341–346. [Google Scholar] [CrossRef]
- Techer, D.; Fontaine, P.; Personne, A.; Viot, S.; Thomas, M. Allelopathic potential and ecotoxicity evaluation of gallic and nonanoic acids to prevent cyanobacterial growth in lentic systems: A preliminary mesocosm study. Sci. Total Environ. 2016, 547, 157–165. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Huang, Q. Allelopathic effects of gallic acid from Aegiceras corniculatum on Cyclotella caspia. J. Environ. Sci. 2013, 25, 776–784. [Google Scholar] [CrossRef]
- Vitalini, S.; Orlando, F.; Palmioli, A.; Alali, S.; Airoldi, C.; De Noni, I.; Vaglia, V.; Bocchi, S.; Iriti, M. Different phytotoxic effect of Lolium multiflorum Lam. leaves against Echinochloa oryzoides (Ard.) Fritsch and Oriza sativa L. Environ. Sci. Pollut. Res. 2020, 27, 33204–33214. [Google Scholar] [CrossRef]
- DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Temussi, F.; Zarrelli, A. New dimeric phenanthrenoids from the rhizomes of Juncus acutus. Structure determination and antialgal activity. Tetrahedron 2003, 59, 2317–2324. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Molinillo, J.M.G. Optimization of Benzoxazinones as Natural Herbicide Models by Lipophilicity Enhancement. J. Agric. Food Chem. 2006, 54, 9357–9365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Zarrelli, A. A new dimeric 9,10-dihydrophenanthrenoid from the rhizome of Juncus acutus. Tetrahedron Lett. 2002, 43, 2573–2575. [Google Scholar] [CrossRef]
- Michalowicz, J.; Duda, W. Phenols sources and toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]






| Tested Species | I50 Values (mg DW Equivalent Extract/mL) | ||
|---|---|---|---|
| Shoot | Root | ||
| Dicot | Alfalfa | 32.6 | 48.0 |
| Cress | 7.8 | 13.9 | |
| Lettuce | 3.3 | 8.7 | |
| Rapeseed | 8.9 | 13.1 | |
| Monocot | Barnyard grass | 42.2 | 16.4 |
| Foxtail fescue | 21.3 | 17.3 | |
| Italian ryegrass | 24.7 | 23.9 | |
| Timothy | 17.1 | 10.4 | |
| Tested Species | Vanillic Acid | Gallic Acid | |
|---|---|---|---|
| (mM) | |||
| Cress | Shoot | 0.04 | 15.4 |
| Root | 0.05 | 13.8 | |
| Italian ryegrass | Shoot | 0.47 | 6.6 |
| Root | 0.45 | 2.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossen, K.; Das, K.R.; Okada, S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic Potential and Active Substances from Wedelia Chinensis (Osbeck). Foods 2020, 9, 1591. https://doi.org/10.3390/foods9111591
Hossen K, Das KR, Okada S, Iwasaki A, Suenaga K, Kato-Noguchi H. Allelopathic Potential and Active Substances from Wedelia Chinensis (Osbeck). Foods. 2020; 9(11):1591. https://doi.org/10.3390/foods9111591
Chicago/Turabian StyleHossen, Kawsar, Krishna Rany Das, Shun Okada, Arihiro Iwasaki, Kiyotake Suenaga, and Hisashi Kato-Noguchi. 2020. "Allelopathic Potential and Active Substances from Wedelia Chinensis (Osbeck)" Foods 9, no. 11: 1591. https://doi.org/10.3390/foods9111591