Revealing Further Insights on Chilling Injury of Postharvest Bananas by Untargeted Lipidomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Lipid Extraction and Analysis
2.3. Lipidomics Analysis
2.4. Ingredients and Relative Contents of Fatty Acids of Membrane Lipids
2.5. PLD and LOX Activities
2.6. Determination of Lipid Peroxidation
2.7. Total RNA Extraction and cDNA Synthesis
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. Lipid Analysis by OPLS-DA Model
3.2. Lipid Constitutes with Significant Differences during Chilling Injury
3.3. Membrane Lipids with Significant Differences during Chilling Injury
3.4. Changes in Fatty Acids Compositions during Chilling Injury
3.5. Changes in Activities and Gene Expressions of Lipid-Related Enzymes during Chilling Injury
3.6. Lipid Peroxidation during Chilling Injury
4. Discussion
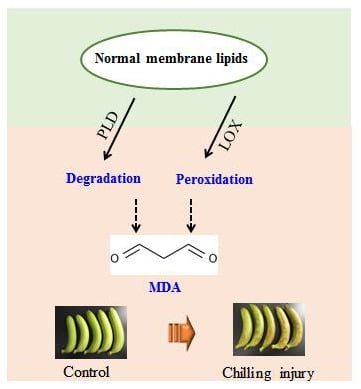
4.1. Chilling Injury of Postharvest Bananas Was Associated with Degradations of Membrane Lipids
4.2. Chilling Injury of Postharvest Bananas Was Associated with Peroxidation of Membrane Lipids
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- John, P.; Marchal, J. Ripening and Biochemistry of the Fruit; Springer: Dordrecht, The Netherlands, 2011; pp. 434–467. [Google Scholar]
- Liu, J.; Li, F.J.; Li, T.T.; Yun, Z.; Duan, X.W.; Jiang, Y.M. Fibroin treatment inhibits chilling injury of banana fruit via energy regulation. Sci. Hortic. 2019, 248, 8–13. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.J.; Liang, L.; Jiang, Y.M.; Chen, J.J. Fibroin delays chilling injury of postharvest banana fruit via enhanced antioxidant capability during cold storage. Metabolites 2019, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, F.J.; Shan, Y.X.; Zhou, Y.J.; Liang, L.; Qu, H.X.; Jiang, Y.M.; Chen, J.J. Determination of H+ and Ca2+ fluxes in cold-stored banana fruit using non-invasive micro-test technology. Postharvest Biol. Technol. 2019, 153, 169–175. [Google Scholar] [CrossRef]
- Holthuis, J.C.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef]
- Kuiper, P.J.C. Lipids in alfalfa leaves in relation to cold hardiness. Plant Physiol. 1970, 45, 684–686. [Google Scholar] [CrossRef]
- Lyons, J.M.; Pratt, W.H.K. Charles albert shull memorial issue || Relationship between the physical nature of mitochondrial membranes and chilling sensitivity in plants. Plant Physiol. 1964, 39, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Malacrida, C.; Valle, E.M.; Boggio, S.B. Postharvest chilling induces oxidative stress response in the dwarf tomato cultivar Micro Tom. Physiol. Plant. 2006, 127, 10–18. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; Seyama, Y.; Shaw, W. A comprehensive classification system for lipids. Eur. J. Lipid Sci. Technol. 2005, 107, 337–364. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef]
- Wang, X.; Chapman, K.D. Lipid signaling in plants. Front Plant Sci. 2013, 4, 216. [Google Scholar] [CrossRef] [Green Version]
- Szymanski, J.; Brotman, Y.; Willmitzer, L.; Cuadros-Inostroza, Á. Linking gene expression and membrane lipid composition of Arabidopsis. Plant Cell 2014, 26, 915–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.; Wei, B.; Gao, Z.; Zhou, Y.; Shi, F.; Zhou, X.; Zhou, Q.; Ji, S. Changes in membrane lipid composition and function accompanying chilling injury in bell peppers. Plant Cell Physiol. 2017, 59, 167–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.S.; Guo, H.M.; Chen, S.Q.; Li, T.T.; Li, M.Q.; Rashid, A.; Xu, C.J.; Wang, K. Methyl jasmonate promotes phospholipid remodeling and jasmonic acid signaling to alleviate chilling injury in peach fruit. J. Agric. Food Chem. 2019, 67, 9958–9966. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yin, X.R.; Zhang, B.; Grierson, D.; Xu, C.J.; Chen, K.S. Transcriptomic and metabolic analyses provide new insights into chilling injury in peach fruit. Plant Cell Environ. 2017, 40, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.E.; Rajashekar, C.B.; Williams, T.W.; Wang, X. Profiling membrane lipids in plant stress responses. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, S.R.; Pandey, S. Phosphatidic acid binding inhibits rgs1 activity to affect specific signaling pathways in Arabidopsis. Plant J. 2017, 90, 466–477. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.L.; Zhou, X.; Sun, H.J.; Zhou, Q.; Wei, B.D.; Cheng, S.C.; Ji, S.J. Glycine betaine treatment alleviates loss of aroma-related esters in cold-store‘Nanguo’ pears by regulating the lipoxygenase pathway. Food Chem. 2020, 316, 126335. [Google Scholar] [CrossRef]
- Ge, W.Y.; Kong, X.M.; Zhao, Y.B.; Wei, B.D.; Cheng, S.C.; Ji, S.J. Insights into the metabolism of membrane lipid fatty acids associated with chilling injury in post-harvest bell peppers. Food Chem. 2019, 295, 26–35. [Google Scholar] [CrossRef]
- Hong, Y.; Jian, Z.; Liang, G.; Kim, S.C.; Deng, X.; Wang, G.; Zhang, G.; Li, M.; Wang, X. Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res. 2016, 62, 55–74. [Google Scholar] [CrossRef] [Green Version]
- Bargmann, B.O.R.; Laxalt, A.M.; Riet, B.T.; Testerink, C.; Merquiol, E.; Mosblech, A.; Leonreyes, A.; Pieterse, C.M.J.; Haring, M.A.; Heilmann, I. Reassessing the role of phospholipase D in the Arabidopsis wounding response. Plant Cell Environ. 2009, 32, 837–850. [Google Scholar] [CrossRef]
- Del Río, L.A.; Sandalio, L.M.; Corpas, F.J.; Palma, J.M.; Barroso, J.B. Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol. 2006, 141, 330–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.S.; Liu, J.H. A Basic helix-loop-helix transcription factor PtrbHLH of Poncirus trifoliata confers cold tolerance and modulates POD-mediated scavenging of H2O2. Plant Physiol. 2013, 162, 1178–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarazona, P.; Feussner, K.; Feussner, I. An enhanced plant lipidomics method based on multiplexed liquid chromatography-mass spectrometry reveals additional insights into cold- and drought-induced membrane remodeling. Plant J. Cell Mol. Biol. 2015, 84, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.L.; Liu, H.B.; Zhang, J.Y.; Lyu, Y.; Li, Z.R.; He, Y.Z.; Zhang, X.L.; Deng, X.X.; Yariv, B.; Fernie, A.R.; et al. Lipidomic and transcriptomic analysis reveals reallocation of carbon flux from cuticular wax into plastid membrane lipids in a glossy “Newhall” navel orange mutant. Hortic. Res. 2020, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Li, C.; Prasad, K.N.; You, X.; Li, L.; Liao, F.; Peng, H.; He, X.; Li, Z.; Zhang, Y. Membrane deterioration, enzymatic browning and oxidative stress in fresh fruits of three litchi cultivars during six-day storage. Sci. Hortic. 2012, 148, 97–103. [Google Scholar] [CrossRef]
- Duan, X.; Liu, T.; Zhang, D.; Su, X.; Lin, H.; Jiang, Y. Effect of pure oxygen atmosphere on antioxidant enzyme and antioxidant activity of harvested litchi fruit during storage. Food Res. Int. 2011, 44, 1905–1911. [Google Scholar] [CrossRef]
- Kuang, J.F.; Chen, J.Y.; Ming, L.; Wu, K.Q.; Wei, S.; Jiang, Y.M.; Lu, W.J. Histone deacetylase HD2 interacts with ERF1 and is involved in longan fruit senescence. J. Exp. Bot. 2012, 63, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Jin, P.; Zhu, H.; Wang, L.; Shan, T.; Zheng, Y. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 2014, 161, 87–93. [Google Scholar] [CrossRef]
- Munnik, T.; Laxalt, A.M. Measuring PLD activity in vivo. Method. Mol. Biol. 2013, 1009, 219–231. [Google Scholar]
- Wang, H.; Qian, Z.; Ma, S.; Zhou, Y.; Patrick, J.W.; Duan, X.; Jiang, Y.; Qu, H. Energy status of ripening and postharvest senescent fruit of litchi (Litchi chinensis Sonn.). BMC Plant Biol. 2013, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, K.; Paliyath, G. Cloning, expression and functional characterization of the C2 domain from tomato phospholipase Dα. Plant Physiol. Biochem. 2011, 49, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Alba-Jiménez, J.E.; Benito-Bautista, P.; Nava, G.M.; Rivera-Pastrana, D.M.; Vázquez-Barrios, M.E.; Mercado-Silva, E.M. Chilling injury is associated with changes in microsomal membrane lipids in guava fruit (Psidium guajava L.) and the use of controlled atmospheres reduce these effects. Sci. Hortic. 2018, 240, 94–101. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Naderi, R.; Jannatizadeh, A.; Sarcheshmeh, M.A.A.; Babalar, M. Enhancement of postharvest chilling tolerance of anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Sci. Hortic. 2016, 198, 52–60. [Google Scholar] [CrossRef]
- Boudière, L.; Michaud, M.; Petroutsos, D.; Rébeillé, F.; Jouhet, J. Glycerolipids in photosynthesis: Composition, synthesis and trafficking. Biochim. Biophys. Acta 2013, 1837, 470–480. [Google Scholar] [CrossRef]
- Gigon, A.; Matos, A.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef]
- Ma, M.J.; Zhu, Z.Q.; Cheng, S.C.; Zhou, Q.; Kong, X.; Hu, M.; Yin, X.C.; Wei, B.D.; Ji, S.J. Methyl jasmonate alleviates chilling injury by regulating membrane lipid composition in green bell pepper. Sci. Hortic. 2020, 266, 109308. [Google Scholar] [CrossRef]
- Huang, H.; Guo, L.F.; Wang, L.; Wang, H.; Ma, S.M.; Jiang, Y.M.; Qu, H.X. 1-Methylcyclopropene (1-MCP) slows ripening of kiwifruit and affects energy status, membrane fatty acid contents and cell membrane integrity. Postharvest Biol. Technol. 2019, 156, 110941. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, S. Crucial contribution of membrane lipids’ unsaturation to acquisition of chilling-tolerance in peach fruit stored at 0 °C. Food Chem. 2009, 115, 405–411. [Google Scholar] [CrossRef]
- Yao, W.; Xu, T.; Farooq, S.U.; Jin, P.; Zheng, Y. Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol. Technol. 2018, 144, 20–28. [Google Scholar] [CrossRef]
- Vazquez-Hernandez, M.; Blanch, M.; Sanchez-Ballesta, M.T.; Merodio, C.; Escribano, M.I. High CO2 alleviates cell ultrastructure damage in Autumn Royal table grapes by modulating fatty acid composition and membrane and cell oxidative status during long-term cold storage. Postharvest Biol. Technol. 2020, 160, 111037. [Google Scholar] [CrossRef]
- He, Y.Z.; Li, Z.R.; Tan, F.Q.; Liu, H.; Zhu, M.; Yang, H.B.; Bi, G.L.; Wan, H.L.; Wang, J.Q.; Xu, R.W. Fatty acid metabolic flux and lipid peroxidation homeostasis maintain the biomembrane stability to improve citrus fruit storage performance. Food Chem. 2019, 292, 314–324. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.J.; Yuan, D.B.; Yun, Z.; Gao, Z.Y.; Su, Z.H.; Zhang, Z.K. Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2020, 160, 111066. [Google Scholar] [CrossRef]
- Liu, H.; Song, L.; You, Y.; Li, Y.; Duan, X.; Jiang, Y.; Joyce, D.C.; Ashraf, M.; Lu, W. Cold storage duration affects litchi fruit quality, membrane permeability, enzyme activities and energy charge during shelf time at ambient temperature. Postharvest Biol. Technol. 2011, 60, 24–30. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Lin, Y.; Zhang, S.; Chen, Y.; Jiang, X. The roles of metabolism of membrane lipids and phenolics in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2016, 111, 51–63. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Z.; Xu, X.; Wang, Q.; Qin, G.; Tian, S. Crucial roles of membrane stability and its related proteins in the tolerance of peach fruit to chilling injury. Amino Acids 2010, 39, 181–194. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Khosravinejad, F.; Hatamnia, A.A.; Sheikhakbari, R.M. Impact of postharvest exogenous γ-aminobutyric acid treatment on cucumber fruit in response to chilling tolerance. Physiol. Mol. Biol. Plants 2017, 23, 827–836. [Google Scholar] [CrossRef]
- Malekzadeh, P. Influence of exogenous application of glycinebetaine on antioxidative system and growth of salt-stressed soybean seedlings (Glycine max L.). Physiol. Mol. Biol. Plants 2015, 21, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Rui, H.; Cao, S.; Shang, H.; Jin, P.; Wang, K.; Zheng, Y. Effects of heat treatment on internal browning and membrane fatty acid in loquat fruit in response to chilling stress. J. Sci. Food Agric. 2010, 90, 1557–1561. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]







| Lipid Class | Fatty Acid | VIP | p Value | Fold Change |
|---|---|---|---|---|
| DG | (18:2/18:2) | 1.40549 | 0.000164291 | 1.992905 |
| DG | (18:3/18:2) | 1.77349 | 0.000211777 | 2.122722 |
| DGDG | (16:0/18:2) | 1.53482 | 0.006909252 | 1.171822 |
| DGDG | (16:0/18:3) | 1.47239 | 0.019451923 | 1.217104 |
| DGDG | (18:3/18:3) | 1.17954 | 0.045065011 | 0.540866 |
| MGDG | (18:1/18:2) | 1.75277 | 0.021060252 | 0.399512 |
| MGDG | (16:0/18:3) | 1.73281 | 0.002636669 | 2.314542 |
| MGDG | (18:2/18:2) | 1.50627 | 0.040431337 | 5.129975 |
| MGDG | (16:1/18:2) | 1.07495 | 0.000376489 | 0.560368 |
| TG | (18:3/18:2/18:3) | 3.77031 | 0.023490239 | 0.074593 |
| TG | (18:3/18:3/18:3) | 3.59434 | 1.34298 × 10−5 | 0.320693 |
| TG | (56:7) | 3.00187 | 2.54755 × 10−6 | 0.207012 |
| TG | (16:0/16:1/18:1) | 1.64902 | 2.62766 × 10−6 | 7.914506 |
| TG | (20:2/18:2/18:2) | 1.22013 | 0.000935517 | 0.138964 |
| PA | (35:2) | 4.22908 | 9.20627 × 10−6 | 0.360367 |
| PA | (18:2/18:2) | 2.13718 | 0.01414934 | 1.637129 |
| PA | (18:3/18:2) | 1.02656 | 0.000960813 | 3.576563 |
| PC | (18:3/18:2) | 11.9358 | 0.014104425 | 131.810267 |
| PC | (18:3/18:3) | 5.75108 | 0.0001374 | 1.872573 |
| PC | (14:0/18:2) | 2.88833 | 0.022713176 | 0.761188 |
| PC | (30:1) | 2.24897 | 0.000328603 | 0.281843 |
| PC | (32:4) | 1.93317 | 0.022367818 | 0.529578 |
| PC | (16:1/18:2) | 1.71136 | 0.00109881 | 0.282682 |
| PC | (16:0/16:1) | 1.45801 | 8.65655 × 10−5 | 0.624131 |
| PC | (38:4) | 1.44706 | 0.004924833 | 55.163991 |
| PE | (16:0/18:2) | 5.66391 | 0.015672899 | 2.638906 |
| PE | (16:0/18:3) | 2.88624 | 0.038558 | 1.779774 |
| PE | (18:3/18:3) | 2.3183 | 3.19625 × 10−5 | 2.353246 |
| PE | (24:0/18:2) | 2.28216 | 0.014856478 | 4.593518 |
| PE | (22:0/18:2) | 1.9654 | 4.62275 × 10−8 | 25.075648 |
| PE | (18:2/18:2) | 1.95415 | 6.73907 × 10−6 | 0.283874 |
| PE | (17:0/18:2) | 1.39272 | 0.027108366 | 1.125154 |
| PE | (18:0/18:2) | 1.3809 | 0.042062853 | 1.182121 |
| PE | (18:0/18:3) | 1.31054 | 0.038018123 | 1.243902 |
| PE | (18:0/18:3) | 1.27068 | 0.003558509 | 1.423061 |
| PE | (18:2/18:2) | 1.18726 | 0.008143585 | 1.155013 |
| PE | (24:0/18:3) | 1.17305 | 0.012577722 | 6.053524 |
| PI | (18:3/18:2) | 1.45654 | 1.75695 × 10−5 | 2.424631 |
| PI | (18:2/18:2) | 1.18232 | 0.000420322 | 1.954515 |
| PG | (16:0/18:2) | 3.0636 | 0.002987396 | 2.016207 |
| PG | (16:0/18:3) | 1.40822 | 0.000845618 | 1.433207 |
| PG | (40:6) | 1.09057 | 0.001160027 | 1.211962 |
| PS | (35:0) | 1.46584 | 5.22552 × 10−5 | 0.585308 |
| LPC | (18:2) | 1.93911 | 0.017220137 | 0.483749 |
| LPE | (18:2) | 1.03778 | 0.002930842 | 0.479456 |
| Category | Fatty Acid Ingredient | Control (%) | Chilling Injury (%) |
|---|---|---|---|
| C18:1 | Oleic acid | 2.40 ± 0.08 a | 1.38 ± 0.10 b |
| C18:2 | Linoleic acid | 4.35 ± 0.02 a | 3.27 ± 0.01 b |
| C18:3 | Linolenic acid | 2.02 ± 0.03 a | 1.02 ± 0.12 b |
| C16:0 | Palmitic acid | 32.11 ± 0.11 b | 36.65 ± 0.18 a |
| C18:0 | Stearic acid | 25.34 ± 0.08 b | 29.88 ± 0.12 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, Q.; Chen, J.; Jiang, Y. Revealing Further Insights on Chilling Injury of Postharvest Bananas by Untargeted Lipidomics. Foods 2020, 9, 894. https://doi.org/10.3390/foods9070894
Liu J, Li Q, Chen J, Jiang Y. Revealing Further Insights on Chilling Injury of Postharvest Bananas by Untargeted Lipidomics. Foods. 2020; 9(7):894. https://doi.org/10.3390/foods9070894
Chicago/Turabian StyleLiu, Juan, Qingxin Li, Junjia Chen, and Yueming Jiang. 2020. "Revealing Further Insights on Chilling Injury of Postharvest Bananas by Untargeted Lipidomics" Foods 9, no. 7: 894. https://doi.org/10.3390/foods9070894