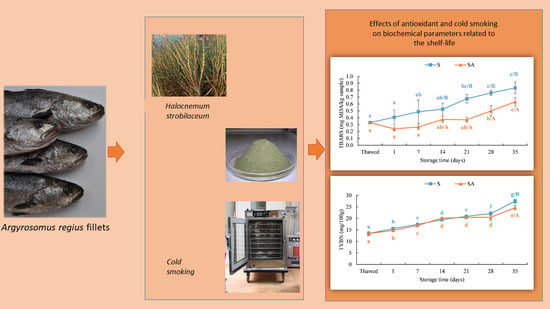
Effect of Cold Smoking and Natural Antioxidants on Quality Traits, Safety and Shelf Life of Farmed Meagre (Argyrosomus regius) Fillets, as a Strategy to Diversify Aquaculture Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Sampling and Processing
2.2. Salting and Smoking
2.3. Physical–Chemical Parameters
2.3.1. Color
2.3.2. Texture Profile Analysis
2.3.3. Water Holding Capacity (WHC)
2.3.4. Muscular pH
2.3.5. Water Activity Determination
2.4. Proximate Composition and Biochemical Parameters Related to the Shelf-Life
2.4.1. Proximate Composition
2.4.2. Biochemical Parameters Related to the Shelf-Life
2.5. Microbiological Analyses
2.6. Sensorial Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physical–Chemical Parameters
3.1.1. Color
3.1.2. Texture Profile Analysis
3.1.3. Water Holding Capacity (WHC)
3.1.4. Muscular pH
3.1.5. Water Activity Determination
3.2. Proximate Composition and Biochemical Parameters Related to the Shelf-Life
3.2.1. Proximate Composition
3.2.2. Biochemical Parameters Related to the Shelf-Life
3.3. Microbiological Analyses
3.4. Sensorial Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Bonanomi, S.; Colombelli, A.; Malvarosa, L.; Cozzolino, M.; Sala, A. Towards the introduction of sustainable fishery products: The bid of a major Italian retailer. Sustainability 2017, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- EUMOFA. The EU Fish Market. European Market Observatory for Fisheries and Aquaculture Products. 2020. Available online: www.eumofa.eu (accessed on 9 July 2021).
- Parisi, G.; Terova, G.; Gasco, L.; Piccolo, G.; Roncarati, A.; Moretti, V.M.; Centoducati, G.; Gatta, P.P.; Pais, A. Current status and future perspectives of Italian finfish aquaculture. Rev. Fish Biol. Fish. 2014, 24, 15–73. [Google Scholar] [CrossRef]
- Poli, B.M.; Parisi, G.; Zampacavallo, G.; Iurzan, F.; Mecatti, M.; Lupi, P.; Bonelli, A. Preliminary results on quality and quality changes in reared meagre (Argyrosomus regius): Body and fillet traits and freshness changes in refrigerated commercial-size fish. Aquac. Int. 2003, 11, 301–311. [Google Scholar] [CrossRef]
- Arab-Tehrany, E.; Jacquot, M.; Gaiani, C.; Imran, M.; Desobry, S.; Linder, M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends Food Sci. Technol. 2012, 25, 24–33. [Google Scholar] [CrossRef]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 8, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallam, K.I. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 2007, 18, 566–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalithapriya, U.; Mariajenita, P.; Renuka, V.; Sudharsan, K.; Karthikeyan, S.; Sivarajan, M.; Murugan, D.; Sukumar, M. Investigation of Natural Extracts and Sodium Bisulfite Impact on Thermal Signals and Physicochemical Compositions of Litopenaeus vannamei during Chilled Storage. J. Aquat. Food Prod. Technol. 2019, 28, 609–623. [Google Scholar] [CrossRef]
- Liau, B.-C.; Shen, C.-T.; Liang, F.-P.; Hong, S.-E.; Hsu, S.-L.; Jong, T.-T.; Chang, C.-M.J. Supercritical fluids extraction and anti-solvent purification of carotenoids from microalgae and associated bioactivity. J. Supercrit Fluids 2010, 55, 169–175. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Hsieh, Y. Distribution of flavonoids and related compounds from seaweeds in Japan. J. Tokyo Univ. Fish. 2003, 89, 1–6. [Google Scholar]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae—A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Anaëlle, T.; Serrano Leon, E.; Laurent, V.; Elena, I.; Mendiola, J.A.; Stéphane, C.; Nelly, K.; Stéphane, L.B.; Luc, M.; Valérie, S.-P. Green improved processes to extract bioactive phenolic compounds from brown macroalgae using Sargassum muticum as model. Talanta 2013, 104, 44–52. [Google Scholar] [CrossRef]
- Burritt, D.J.; Larkindale, J.; Hurd, C.L. Antioxidant metabolism in the intertidal red seaweed Stictosiphonia arbuscula following desiccation. Planta 2002, 215, 829–838. [Google Scholar] [CrossRef]
- Hassoun, A.; Emir Çoban, Ö. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends Food Sci. Technol. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Licciardello, F.; Siracusa, L.; Muratore, G.; Hamdi, M.; Restuccia, C. Antimicrobial and antioxidant features of ‘Gabsiʼ pomegranate peel extracts. Ind. Crops Prod. 2018, 111, 345–352. [Google Scholar] [CrossRef]
- Messina, C.M.; Renda, G.; Laudicella, V.A.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A. From ecology to biotechnology, study of the defense strategies of algae and halophytes (from Trapani Saltworks, NW Sicily) with a focus on antioxidants and antimicrobial properties. Int. J. Mol. Sci. 2019, 20, 881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, C.M.; Bono, G.; Renda, G.; La Barbera, L.; Santulli, A. Effect of natural antioxidants and modified atmosphere packaging in preventing lipid oxidation and increasing the shelf-life of common dolphinfish (Coryphaena hippurus) fillets. LWT—Food Sci. Technol. 2015, 62, 271–277. [Google Scholar] [CrossRef]
- Messina, C.M.; Bono, G.; Arena, R.; Randazzo, M.; Morghese, M.; Manuguerra, S.; La Barbera, L.; Ozogul, F.; Sadok, S.; Santulli, A. The combined impact of cold smoking and natural antioxidants on quality and shelf life of dolphinfish (Coryphaena hippurus) fillets. Food Sci. Nutr. 2019, 7, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Sampels, S. The effects of processing technologies and preparation on the final quality of fish products. Trends Food Sci. Technol. 2015, 44, 131–146. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kotsanopoulos, K. V Smoking of Fish and Seafood: History, Methods and Effects on Physical, Nutritional and Microbiological Properties. Food Bioprocess Technol. 2012, 5, 831–853. [Google Scholar] [CrossRef]
- Messina, C.M.; Arena, R.; Ficano, G.; La Barbera, L.; Morghese, M.; Santulli, A. Combination of Freezing, Low Sodium Brine, and Cold Smoking on the Quality and Shelf-Life of Sea Bass (Dicentrarchus labrax L.) Fillets as a Strategy to Innovate the Market of Aquaculture Products. Animals 2021, 11, 185. [Google Scholar] [CrossRef]
- Birkeland, S.; Bjerkeng, B. The quality of cold-smoked Atlantic salmon (Salmo salar) as affected by salting method, time and temperature. Int. J. Food Sci. Technol. 2005, 40, 963–976. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Giménez, B.; Gómez-Guillén, C.; Montero, P. Influence of frozen storage on aptitude of sardine and dolphinfish for cold-smoking process. LWT—Food Sci. Technol. 2010, 43, 1246–1252. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Gómez-Estaca, J.; Giménez, B.; Montero, P. Alternative fish species for cold-smoking process. Int. J. Food Sci. Technol. 2009, 44, 1525–1535. [Google Scholar] [CrossRef] [Green Version]
- Cardinal, M.; Cornet, J.; Serot, T.; Baron, R. Effects of the smoking process on odour characteristics of smoked herring (Clupea harengus) and relationships with phenolic compound content. Food Chem. 2006, 96, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Montero, P. The effect of combined traditional and novel treatments on oxidative status of dolphinfish (Coryphaena hippurus) and sardine (Sardina pilchardus) muscle lipids. Food Sci. Technol. Int. 2013, 20, 431–440. [Google Scholar] [CrossRef]
- Puke, S.; Galoburda, R. Factors affecting smoked fish quality: A review. In Proceedings of the Research for Rural Development, Jelgava, Latvia, 13–15 May 2020; Volume 35, pp. 132–139. [Google Scholar]
- Sung, J.-H.; Park, S.-H.; Seo, D.-H.; Lee, J.-H.; Hong, S.-W.; Hong, S.-S. Antioxidative and Skin-Whitening Effect of an Aqueous Extract of Salicornia herbacea. Biosci. Biotechnol. Biochem. 2009, 73, 552–556. [Google Scholar] [CrossRef] [Green Version]
- CIE. Commission Internationale de l’Eclairage Colorimetry, 3rd ed.; CIE: Vienna, Austria, 2004; Volume 15, pp. 1–82. [Google Scholar]
- Commission Internationale de l’Eclairage. CIE Recommendations on Uniform Color Spaces, Color-Difference Equations, and Metric Color Terms. Color Res. Appl. 1977, 2, 5–6. [Google Scholar] [CrossRef]
- Robertson, A.R. Historical development of CIE recommended color difference equations. Color Res. Appl. 1990, 15, 167–170. [Google Scholar] [CrossRef]
- Messina, C.M.; Arena, R.; Manuguerra, S.; Renda, G.; Laudicella, V.A.; Ficano, G.; Fazio, G.; La Barbera, L.; Santulli, A. Farmed Gilthead Sea Bream (Sparus aurata) by-Products Valorization: Viscera Oil ω-3 Enrichment by Short-Path Distillation and In Vitro Bioactivity Evaluation. Mar. Drugs 2021, 19, 160. [Google Scholar] [CrossRef]
- Orban, E.; Sinesio, F.; Paoletti, F. The Functional Properties of the Proteins, Texture and the Sensory Characteristics of Frozen Sea Bream Fillets (Sparus aurata) from different farming systems. LWT—Food Sci. Technol. 1997, 30, 214–217. [Google Scholar] [CrossRef]
- Teixeira, B.; Fidalgo, L.; Mendes, R.; Costa, G.; Cordeiro, C.; Marques, A.; Saraiva, J.A.; Nunes, M.L. Effect of high pressure processing in the quality of sea bass (Dicentrarchus labrax) fillets: Pressurization rate, pressure level and holding time. Innov. Food Sci. Emerg. Technol. 2014, 22, 31–39. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting; AOAC: Rockville, MD, USA, 1990; Volume 15, ISBN 9780935584752. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). AOAC Official Method 981.10 Crude Protein in Meat Block Digestion Method; AOAC: Rockville, MD, USA, 1992; Volume 65, p. 1339. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Messina, C.M.; Renda, G.; La Barbera, L.; Santulli, A. By-products of farmed European sea bass (Dicentrarchus labrax L.) as a potential source of n-3 PUFA. Biologia 2013, 68, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, Sensitive, and Specific Thiobarbituric Acid Method for Measuring Lipid Peroxidation in Animal Tissue, Food, and Feedstuff Samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Commissione delle Comunità Europee. 95/149/CE: Decisione della Commissione, dell’8 marzo 1995, che fissa i Valori Limite di ABVT (Azoto Basico Volatile Totale) per Talune Categorie di Prodotti della Pesca e i Relativi Metodi D’analisi; Commissione delle Comunità Europee: Bruxelles, Belgium, 1995. [Google Scholar]
- Bilgin, Ş.; Değirmenci, A. Quality changes in reared, hot-smoked meagre (Argyrosomus regius asso, 1801) during chill storage at 4 ± 1 °C. Food Sci. Technol. 2019, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, A.; Fernandez-Segovia, I.; Barat, J.M.; Serra, J.A. Physicochemical characterization of some smoked and marinated fish products. J. Food Process. Preserv. 2010, 34, 83–103. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Zeng, X.A.; Liu, D. Recent Advances in Methods and Techniques for Freshness Quality Determination and Evaluation of Fish and Fish Fillets: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1012–1225. [Google Scholar] [CrossRef]
- Choubert, G.; Blanc, J.-M.; Courvalin, C. Muscle carotenoid content and colour of farmed rainbow trout fed astaxanthin or canthaxanthin as affected by cooking and smoke-curing procedures. Int. J. Food Sci. Technol. 1992, 27, 277–284. [Google Scholar] [CrossRef]
- Cardinal, M.; Knockaert, C.; Torrissen, O.; Sigurgisladottir, S.; Morkore, T.; Thomassen, M.; Vallet, J.L. Relation of smoking parameters to the yield, colour and sensory quality of smoked Atlantic salmon (Salmo salar). Food Res. Int. 2001, 34, 537–550. [Google Scholar] [CrossRef] [Green Version]
- Birkeland, S.; Rørå, A.M.B.; Skåra, T.; Bjerkeng, B. Effects of cold smoking procedures and raw material characteristics on product yield and quality parameters of cold smoked Atlantic salmon (Salmo salar L.) fillets. Food Res. Int. 2004, 37, 273–286. [Google Scholar] [CrossRef]
- Offer, G.; Knight, P.; Jeacocke, R.; Almond, R.; Cousins, T. The structural basis of the water-holding, appearance and toughness of meat and meat products. Food Microstruct. 1989, 8, 151–170. [Google Scholar]
- Bekhit, A.E.D.A.; Morton, J.D.; Dawson, C.O.; Sedcole, R. Optical properties of raw and processed fish roes from six commercial New Zealand species. J. Food Eng. 2009, 91, 363–371. [Google Scholar] [CrossRef]
- Chan, S.S.; Roth, B.; Jessen, F.; Løvdal, T.; Jakobsen, A.N.; Lerfall, J. A comparative study of Atlantic salmon chilled in refrigerated seawater versus on ice: From whole fish to cold-smoked fillets. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Richards, M.P.; Hultin, H.O. Effect of pH on lipid oxidation using trout hemolysate as a catalyst: A possible role for deoxyhemoglobin. J. Agric. Food Chem. 2000, 48, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.; Fernández-Segovia, I.; Serra, J.A.; Barat, J.M. Development of a smoked sea bass product with partial sodium replacement. LWT—Food Sci. Technol. 2010, 43, 1426–1433. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernandez-Segovia, I.; Serra, J.A.; Barat, J.M. Effect of partial sodium replacement on physicochemical parameters of smoked sea bass during storage. Food Sci. Technol. Int. 2012, 18, 207–217. [Google Scholar] [CrossRef]
- Regost, C.; Jakobsen, J.V.; Rørå, A.M.B. Flesh quality of raw and smoked fillets of Atlantic salmon as influenced by dietary oil sources and frozen storage. Food Res. Int. 2004, 37, 259–271. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Montero, P.; Hurtado, O.; Borderías, A.J. Biological characteristics affect the quality of farmed atlantic salmon and smoked muscle. J. Food Sci. 2000, 65, 53–60. [Google Scholar] [CrossRef]
- Sigurgisladottir, S.; Sigurdardottir, M.S.; Torrissen, O.; Vallet, J.L.; Hafsteinsson, H. Effects of different salting and smoking processes on the microstructure, the texture and yield of Atlantic salmon (Salmo salar) fillets. Food Res. Int. 2000, 33, 847–855. [Google Scholar] [CrossRef]
- Hernández, M.D.; Martínez, F.J.; García García, B. Sensory evaluation of farmed sharpsnout seabream (Diplodus puntazzo). Aquac. Int. 2001, 9, 519–529. [Google Scholar] [CrossRef]
- Liu, S.; Fan, W.; Zhong, S.; Ma, C.; Li, P.; Zhou, K.; Peng, Z.; Zhu, M. Quality evaluation of tray-packed tilapia fillets stored at 0 °C based on sensory, microbiological, biochemical and physical attributes. Afr. J. Biotechnol. 2010, 9, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Huff-lonergan, E.; Lonergan, S.M. MEAT Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Chan, S.S.; Roth, B.; Skare, M.; Hernar, M.; Jessen, F.; Løvdal, T.; Jakobsen, A.N.; Lerfall, J. Effect of chilling technologies on water holding properties and other quality parameters throughout the whole value chain: From whole fish to cold-smoked fillets of Atlantic salmon (Salmo salar). Aquaculture 2020, 526, 735381. [Google Scholar] [CrossRef]
- Hassan, I.M. Processing of smoked common carp fish and its relation to some chemical, physical and organoleptic properties. Food Chem. 1988, 27, 95–106. [Google Scholar] [CrossRef]
- Leroi, F.; Joffraud, J.J. Salt and smoke simultaneously affect chemical and sensory quality of cold-smoked salmon during 5 degrees C storage predicted using factorial design. J. Food Prot. 2000, 63, 1222–1227. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Sensory and biochemical aspects of quality of whole bigeye tuna (Thunnus obesus) during bulk storage in controlled atmospheres. Food Chem. 2005. [Google Scholar] [CrossRef]
- Osheba, A.S. Technological attempts for production of low sodium smoked herring fish (Renga). Adv. J. Food Sci. Technol. 2013, 5, 695–706. [Google Scholar] [CrossRef]
- Oliveira, H.; Pedro, S.; Nunes, M.L.; Costa, R.; Vaz-Pires, P. Processing of Salted Cod (Gadus spp.): A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 546–564. [Google Scholar] [CrossRef]
- Tang, S.; Kerry, J.P.; Sheehan, D.; Buckley, D.J.; Morrissey, P.A. Antioxidative effect of added tea catechins on susceptibility of cooked red meat, poultry and fish patties to lipid oxidation. Food Res. Int. 2001, 34, 651–657. [Google Scholar] [CrossRef]
- Grigorakis, K.; Fountoulaki, E.; Vasilaki, A.; Mittakos, I.; Nathanailides, C. Lipid quality and filleting yield of reared meagre (Argyrosomus regius). Int. J. Food Sci. Technol. 2011, 46, 711–716. [Google Scholar] [CrossRef]
- Bozova, B.; İzci, L. Acta Aquatica Turcica Effects of Plant Extracts on the Quality of Sous Vide Meagre (Argyrosomus regius) Fillets. Acta Aquatica Turcica 2021, 17, 255–266. [Google Scholar]
- Hussain, M.A.; Sumon, T.A.; Mazumder, S.K.; Ali, M.M.; Jang, W.J.; Abualreesh, M.H.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, H.T.; Lee, E.W.; et al. Essential oils and chitosan as alternatives to chemical preservatives for fish and fisheries products: A review. Food Control 2021, 129, 108244. [Google Scholar] [CrossRef]
- Leistner, L.; Gorris, L.G.M. Food preservation by hurdle technology. Trends Food Sci. Technol. 1995, 6, 41–46. [Google Scholar] [CrossRef]
- Özyurt, G.; Kuley, E.; Özkütük, S.; Özogul, F. Sensory, microbiological and chemical assessment of the freshness of red mullet (Mullus barbatus) and goldband goatfish (Upeneus moluccensis) during storage in ice. Food Chem. 2009, 114, 505–510. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernández-Segovia, I.; Barat, J.M.; Serra, J.A. Influence of sodium replacement and packaging on quality and shelf life of smoked sea bass (Dicentrarchus labrax L.). LWT—Food Sci. Technol. 2011, 44, 917–923. [Google Scholar] [CrossRef]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- AFSSA. AVIS de l’Agence Française de Sécurité Sanitaire des Aliments Concernant les Références Applicables aux Denrées Alimentaires en tant que Critères Indicateurs D’hygiène des Procédés; AFSSA: Maisons-Alfort, France, 2008; pp. 1–21. [Google Scholar]
- FCD. Critères Microbiologiques Applicables à Partir de 2010 aux Marques de Distributeurs, Marques Premiers prix et Matières Premières dans leur Conditionnement Initial Industriel; FCD: Paris, France, 2009; pp. 1–57. [Google Scholar]
- Healt Protection Agency. Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods Placed on the Market; Healt Protection Agency: London, UK, 2009. [Google Scholar]
- Decreto Ministeriale del 18/09/2002. Modalità di Informazione sullo Stato di Qualità delle Acque, del Decreto Legislativo 11 Maggio 1999, n. 152. Gazz. Uff. Suppl. Ordin. n° 245 del 18/10/2002; Ministro dell’Ambiente: Rome, Italy, 2002; p. 31030.
- D.S. Reg. Piemonte Allegato B Linee Guida per l’analisi del Rischio. Progett. Reg. “Analisi del Rischio Microbiol. Legato al Consum. di Aliment. Final. alla Riduzione dei Costi Anal. Approv. con Determ. della Dir. Sanità della Reg. Piemonte n.780 del 18 Ottobre 2011. 2013, pp. 1–41. Available online: http://www.regione.piemonte.it/governo/bollettino/abbonati/2011/51/attach/dddb200000780_830.pdf (accessed on 1 September 2021).
- Messina, C.M.; Bono, G.; Arena, R.; Randazzo, M.; Manuguerra, S.; Santulli, A. Polyphenols from halophytes and modified atmosphere packaging improve sensorial and biochemical markers of quality of common dolphinfish (Coryphaena hippurus) fillets. Food Sci. Nutr. 2016, 4, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Capillas, C.; Moral, A. Residual effect of CO2 on hake (Merluccius merluccius L.) stored in modified and controlled atmospheres. Eur. Food Res. Technol. 2001, 212, 413–420. [Google Scholar] [CrossRef]










| Storage Time (Days) | S | SA | |
|---|---|---|---|
| L* (D65) | Thawed | 45.48 ± 1.43 c | 44.65 ± 1.41 d |
| 0 | 40.86 ± 1.26 a | 39.87 ± 1.13 b | |
| 1 | 40.52 ± 0.57 a | 39.46 ± 0.18 b | |
| 7 | 39.17 ± 0.67 a | 38.87 ± 0.10 ab | |
| 14 | 40.96 ± 1.03 a/B | 37.96 ± 0.41 a/A | |
| 21 | 40.14 ± 0.18 a/B | 38.36 ± 0.07 a/A | |
| 28 | 39.90 ± 0.76 a | 39.84 ± 0.59 b | |
| 35 | 42.72 ± 0.33 b/B | 41.38 ± 0.39 c/A | |
| a* (D65) | Thawed | −2.12 ± 0.39 d | −2.31 ± 0.43 c |
| 0 | −2.35 ± 0.28 cd | −2.72 ± 0.31 bc | |
| 1 | −2.57 ± 0.07 c/B | −2.85 ± 0.10 bc/A | |
| 7 | −3.47 ± 0.36 b | −3.55 ± 0.12 ab | |
| 14 | −3.29 ± 0.03 b | −2.63 ± 0.79 b | |
| 21 | −4.31 ± 0.02 a/A | −3.50 ± 0.10 ab/B | |
| 28 | −3.61 ± 0.06 b/A | −3.09 ± 0.09 b/B | |
| 35 | −3.31 ± 0.39 b/B | −4.25 ± 0.10 a/A | |
| b* (D65) | Thawed | −3.56 ± 0.83 b | −2.61 ± 0.75 a |
| 0 | −6.67 ± 0.59 a/A | −3.90 ± 0.68 a/B | |
| 1 | −5.62 ± 0.63 b/A | −2.80 ± 0.58 a/B | |
| 7 | −3.41 ± 0.96 c/A | −1.05 ± 0.01 b/B | |
| 14 | −2.20 ± 0.13 cd/A | 0.37 ± 0.37 c/B | |
| 21 | −0.75 ± 0.64 d | 0.43 ± 0.22 c | |
| 28 | −0.14 ± 0.54 d | 1.41 ± 0.59 c | |
| 35 | −1.68 ± 0.79 cd/A | 1.51 ± 0.08 c/B | |
| C* (D65) | Thawed | 4.21 ± 0.84 a | 3.53 ± 0.82 ab |
| 0 | 7.09 ± 0.62 b/B | 4.78 ± 0.69 b/A | |
| 1 | 6.25 ± 0.53 b/B | 4.04 ± 0.47 ab/A | |
| 7 | 4.91 ± 0.92 a | 3.82 ± 0.05 ab | |
| 14 | 4.16 ± 0.23 a | 2.81 ± 0.67 a | |
| 21 | 4.53 ± 0.16 a/B | 3.68 ± 0.16 ab/A | |
| 28 | 3.80 ± 0.01 a | 3.50 ± 0.31 ab | |
| 35 | 3.93 ± 0.01 a/A | 4.60 ± 0.14 b/B | |
| h (D65) | Thawed | 238.17 ± 4.55 d | 226.24 ± 5.82 cd |
| 0 | 250.57 ± 1.60 e/B | 234.51 ± 3.37 d/A | |
| 1 | 244.05 ± 2.92 d/B | 222.83 ± 5.22 c/A | |
| 7 | 222.51 ± 5.36 c/B | 195.21 ± 0.43 b/A | |
| 14 | 211.64 ± 0.05 bc/B | 167.86 ± 10.09 a/A | |
| 21 | 188.24 ± 7.93 a | 172.47 ± 3.32 a | |
| 28 | 180.02 ± 8.95 ab | 156.66 ± 8.67 a | |
| 35 | 206.35 ± 13.97 bc/B | 161.40 ± 0.16 a/A |
| Storage Time (Days) | S | SA | |
|---|---|---|---|
| Total Lipids | Thawed | 0.85 ± 0.06 a | 0.88 ± 0.12 a |
| 1 | 1.72 ± 0.26 b | 2.15 ± 0.23 b | |
| 35 | 2.25 ± 0.26 b | 2.51 ± 0.39 b | |
| Moisture | Thawed | 79.57 ± 1.74 b | 80.57 ± 1.79 b |
| 1 | 74.96 ± 1.54 a | 74.19 ± 1.05 a | |
| 35 | 73.03 ± 1.17 a | 73.08 ± 1.71 a | |
| Protein | Thawed | 18.06 ± 1.24 | 17.04 ± 1.18 |
| 1 | 18.18 ± 0.10 | 18.02 ± 0.10 | |
| 35 | 18.84 ± 0.53 | 18.84 ± 0.81 | |
| Ash | Thawed | 1.26 ± 0.07 a | 1.23 ± 0.07 a |
| 1 | 5.13 ± 0.88 b | 5.22 ± 0.68 b | |
| 35 | 5.10 ± 0.15 b | 4.67 ± 0.88 b |
| Days | S | SA | |
|---|---|---|---|
| Appearance | 1 | 9.00 ± 0.82 bcd | 9.00 ± 0.00 bcd |
| 7 | 10.00 ± 0.00 d | 9.25 ± 0.50 d | |
| 14 | 8.25 ± 0.50 abc | 8.75 ± 0.50 bcd | |
| 21 | 8.00 ± 1.15 abc | 7.75 ± 0.50 ab | |
| 28 | 8.00 ± 0.89 abc | 8.17 ± 0.41 abc | |
| 35 | 7.17 ± 0.75 a | 7.00 ± 0.89 a | |
| Color | 1 | 9.25 ± 0.96 cd | 8.75 ± 0.50 bc |
| 7 | 10.00 ± 0.00 d | 9.25 ± 0.50 c | |
| 14 | 8.50 ± 0.58 bc | 8.75 ± 0.50 bc | |
| 21 | 8.50 ± 0.58 bc | 8.25 ± 0.96 b | |
| 28 | 8.17 ± 0.98 bc | 8.50 ± 0.55 b | |
| 35 | 7.33 ± 0.52 ab | 7.00 ± 0.89 a | |
| Odor | 1 | 9.50 ± 0.58 d | 8.00 ± 0.00 b |
| 7 | 9.50 ± 0.58 d | 9.25 ± 0.50 b | |
| 14 | 8.50 ± 0.58 bcd | 9.00 ± 0.82 b | |
| 21 | 8.50 ± 0.58 bcd | 8.00 ± 0.82 b | |
| 28 | 7.83 ± 0.75 bc | 8.17 ± 0.41 b | |
| 35 | 7.17 ± 0.75 ab | 6.67 ± 1.03 a | |
| Texture | 1 | 9.75 ± 0.50 c | 7.50 ± 0.58 a |
| 7 | 9.75 ± 0.50 c | 9.00 ± 0.00 bc | |
| 14 | 9.00 ± 0.00 bc | 9.75 ± 0.50 c | |
| 21 | 8.50 ± 0.58 b | 8.25 ± 0.96 b | |
| 28 | 9.00 ± 0.00 bc | 9.00 ± 0.00 bc | |
| 35 | 7.33 ± 0.52 a | 7.00 ± 0.89 a | |
| Taste | 1 | 9.50 ± 0.58 b | 8.25 ± 0.50 bc |
| 7 | 9.50 ± 0.58 b | 9.25 ± 0.50 c | |
| 14 | 8.50 ± 0.58 ab | 9.00 ± 0.82 c | |
| 21 | 8.25 ± 0.96 ab | 7.25 ± 0.96 ab | |
| 28 | 7.83 ± 0.75 a | 8.17 ± 0.41 bc | |
| 35 | 7.17 ± 0.75 a | 6.67 ± 1.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messina, C.M.; Arena, R.; Ficano, G.; Randazzo, M.; Morghese, M.; La Barbera, L.; Sadok, S.; Santulli, A. Effect of Cold Smoking and Natural Antioxidants on Quality Traits, Safety and Shelf Life of Farmed Meagre (Argyrosomus regius) Fillets, as a Strategy to Diversify Aquaculture Products. Foods 2021, 10, 2522. https://doi.org/10.3390/foods10112522
Messina CM, Arena R, Ficano G, Randazzo M, Morghese M, La Barbera L, Sadok S, Santulli A. Effect of Cold Smoking and Natural Antioxidants on Quality Traits, Safety and Shelf Life of Farmed Meagre (Argyrosomus regius) Fillets, as a Strategy to Diversify Aquaculture Products. Foods. 2021; 10(11):2522. https://doi.org/10.3390/foods10112522
Chicago/Turabian StyleMessina, Concetta Maria, Rosaria Arena, Giovanna Ficano, Mariano Randazzo, Maria Morghese, Laura La Barbera, Saloua Sadok, and Andrea Santulli. 2021. "Effect of Cold Smoking and Natural Antioxidants on Quality Traits, Safety and Shelf Life of Farmed Meagre (Argyrosomus regius) Fillets, as a Strategy to Diversify Aquaculture Products" Foods 10, no. 11: 2522. https://doi.org/10.3390/foods10112522