Shedding Light on Bladder Cancer Diagnosis in Urine
Abstract
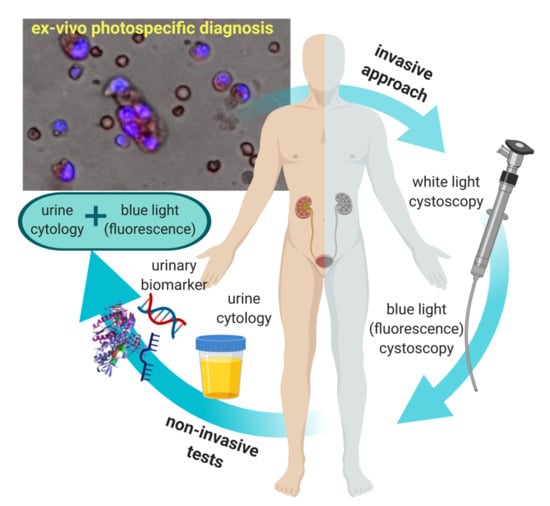
:1. Introduction
2. Current Noninvasive Test for Bladder Cancer Diagnosis Targeting Urinary Biomarkers
3. Current Use of Photodynamic Diagnostic for Bladder Cancer Detection
4. Use of Photosensitiser Prodrug in Urine Towards Noninvasive Diagnostic
4.1. 5-ALA-based Approaches
4.2. Hypericin-Based Approaches
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int. J. Cancer 2018, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berdik, C. Unlocking bladder cancer. Nature 2017, 551, S34. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Böhle, A.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Hernández, V.; Kaasinen, E.; Palou, J.; Rouprêt, M.; et al. Eau guidelines on non muscle-invasive urothelial carcinoma of the bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Kostirova, M.; Mudra, K.; Pecher, S.; Smolova, H.; Pecen, L.; Ibrahim, Z.; Dvoracek, J.; Jarolim, L.; Novak, J.; et al. Qualitative and quantitative detection of urinary human complement factor h-related protein (bta stat and bta trak) and fragments of cytokeratins 8, 18 (ubc rapid and ubc irma) as markers for transitional cell carcinoma of the bladder. Eur. Urol. 2002, 41, 34–39. [Google Scholar] [CrossRef]
- Rink, M.; Babjuk, M.; Catto, J.W.; Jichlinski, P.; Shariat, S.F.; Stenzl, A.; Stepp, H.; Zaak, D.; Witjes, J.A. Hexyl aminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: A critical review of the current literature. Eur. Urol. 2013, 64, 624–638. [Google Scholar] [CrossRef]
- Jichlinski, P.; Guillou, L.; Karlsen, S.J.; MalmstrÖM, P.-U.; Jocham, D.; Brennhovd, B.; Johansson, E.V.A.; GÄRtner, T.; Lange, N.; van den Bergh, H.; et al. Hexyl aminolevulinate fluorescence cystoscopy: A new diagnostic tool for photodiagnosis of superficial bladder cancer—A multicenter study. J. Urol. 2003, 170, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Geavlete, B.; Multescu, R.; Georgescu, D.; Jecu, M.; Stanescu, F.; Geavlete, P. Treatment changes and long-term recurrence rates after hexaminolevulinate (hal) fluorescence cystoscopy: Does it really make a difference in patients with non-muscle-invasive bladder cancer (nmibc)? BJU Int. 2012, 109, 549–556. [Google Scholar] [CrossRef]
- Jocham, D.; Witjes, F.; Wagner, S.; Zeylemaker, B.; van Moorselaar, J.; Grimm, M.-O.; Muschter, R.; Popken, G.; KöNig, F.; KnüChel, R.; et al. Improved detection and treatment of bladder cancer using hexaminolevulinate imaging: A prospective, phase iii multicenter study. J. Urol. 2005, 174, 862–866. [Google Scholar] [CrossRef] [Green Version]
- Schmidbauer, J.; Witjes, F.; Schmeller, N.; Donat, R.; Susani, M.; Marberger, M. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J. Urol. 2004, 171, 135–138. [Google Scholar] [CrossRef]
- Jordan, B.; Meeks, J.J. T1 bladder cancer: Current considerations for diagnosis and management. Nat. Rev. Urol. 2019, 16, 23–34. [Google Scholar] [CrossRef]
- Bach, T.; Bastian, P.J.; Blana, A.; Kaminsky, A.; Keller, S.; Knoll, T.; Lang, C.; Promnitz, S.; Ubrig, B.; Keller, T.; et al. Optimised photodynamic diagnosis for transurethral resection of the bladder (turb) in german clinical practice: Results of the noninterventional study optic iii. World J. Urol. 2017, 35, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and treatment of non-muscle invasive bladder cancer: Aua/suo guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Grossman, H.B.; Droller, M.; Schmidbauer, J.; Hermann, G.; Drăgoescu, O.; Ray, E.; Fradet, Y.; Karl, A.; Burgués, J.P.; et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: A meta-analysis of detection and recurrence based on raw data. Eur. Urol. 2013, 64, 846–854. [Google Scholar] [CrossRef]
- Daneshmand, S.; Patel, S.; Lotan, Y.; Pohar, K.; Trabulsi, E.; Woods, M.; Downs, T.; Huang, W.; Jones, J.; O’Donnell, M.; et al. Efficacy and safety of blue light flexible cystoscopy with hexaminolevulinate in the surveillance of bladder cancer: A phase iii, comparative, multicenter study. J. Urol. 2018, 199, 1158–1165. [Google Scholar] [CrossRef]
- Chakraborty, A.; Dasari, S.; Long, W.; Mohan, C. Urine protein biomarkers for the detection, surveillance, and treatment response prediction of bladder cancer. Am. J. Cancer Res. 2019, 9, 1104–1117. [Google Scholar]
- Lintula, S.; Hotakainen, K. Developing biomarkers for improved diagnosis and treatment outcome monitoring of bladder cancer. Exp. Opin. Biol. Ther. 2010, 10, 1169–1180. [Google Scholar] [CrossRef]
- Maas, M.; Walz, S.; Stuhler, V.; Aufderklamm, S.; Rausch, S.; Bedke, J.; Stenzl, A.; Todenhofer, T. Molecular markers in disease detection and follow-up of patients with non-muscle invasive bladder cancer. Exp. Rev. Mol. Diagn. 2018, 18, 443–455. [Google Scholar] [CrossRef]
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Grusing, S.; Selph, S. Urinary biomarkers for diagnosis of bladder cancer: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 163, 922–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousef, P.G.; Gabril, M.Y. An update on the molecular pathology of urinary bladder tumors. Pathol.—Res. Pract. 2018, 214, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Karam, J.A.; Grossman, H.B.; Kader, A.K.; Munsell, M.; Dinney, C.P. Prospective trial to identify optimal bladder cancer surveillance protocol: Reducing costs while maximizing sensitivity. BJU Int. 2011, 108, 1119–1123. [Google Scholar] [CrossRef]
- Shen, C.; Sun, Z.; Chen, D.; Su, X.; Jiang, J.; Li, G.; Lin, B.; Yan, J. Developing urinary metabolomic signatures as early bladder cancer diagnostic markers. Omics 2015, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frantzi, M.; van Kessel, K.E.; Zwarthoff, E.C.; Marquez, M.; Rava, M.; Malats, N.; Merseburger, A.S.; Katafigiotis, I.; Stravodimos, K.; Mullen, W.; et al. Development and validation of urine-based peptide biomarker panels for detecting bladder cancer in a multi-center study. Clin Cancer Res. 2016, 22, 4077–4086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinges, S.S.; Hohm, A.; Vandergrift, L.A.; Nowak, J.; Habbel, P.; Kaltashov, I.A.; Cheng, L.L. Cancer metabolomic markers in urine: Evidence, techniques and recommendations. Nat. Rev. Urol. 2019, 16, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Tan, W.P.; Tan, M.-Y.; Khetrapal, P.; Dong, L.; deWinter, P.; Feber, A.; Kelly, J.D. Novel urinary biomarkers for the detection of bladder cancer: A systematic review. Cancer Treat. Rev. 2018, 69, 39–52. [Google Scholar] [CrossRef]
- Huang, Y.L.; Chen, J.; Yan, W.; Zang, D.; Qin, Q.; Deng, A.M. Diagnostic accuracy of cytokeratin-19 fragment (cyfra 21–1) for bladder cancer: A systematic review and meta-analysis. Tumour Biol 2015, 36, 3137–3145. [Google Scholar] [CrossRef]
- Konety, B.R.; Nguyen, T.S.; Brenes, G.; Sholder, A.; Lewis, N.; Bastacky, S.; Potter, D.M.; Getzenberg, R.H. Clinical usefulness of the novel marker blca-4 for the detection of bladder cancer. J. Urol. 2000, 164, 634–639. [Google Scholar] [CrossRef]
- Shariat, S.F.; Casella, R.; Khoddami, S.M.; Hernandez, G.; Sulser, T.; Gasser, T.C.; Lerner, S.P. Urine detection of survivin is a sensitive marker for the noninvasive diagnosis of bladder cancer. J. Urol. 2004, 171, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; O’Sullivan, P.; Raman, J.D.; Shariat, S.F.; Kavalieris, L.; Frampton, C.; Guilford, P.; Luxmanan, C.; Suttie, J.; Crist, H.; et al. Clinical comparison of noninvasive urine tests for ruling out recurrent urothelial carcinoma. Urol. Oncol. 2017, 35, 531.e515–531.e522. [Google Scholar] [CrossRef]
- Beukers, W.; van der Keur, K.A.; Kandimalla, R.; Vergouwe, Y.; Steyerberg, E.W.; Boormans, J.L.; Jensen, J.B.; Lorente, J.A.; Real, F.X.; Segersten, U.; et al. Fgfr3, tert and otx1 as a urinary biomarker combination for surveillance of patients with bladder cancer in a large prospective multicenter study. J. Urol. 2017, 197, 1410–1418. [Google Scholar] [CrossRef]
- Wallace, E.; Higuchi, R.; Satya, M.; McCann, L.; Sin, M.L.Y.; Bridge, J.A.; Wei, H.; Zhang, J.; Wong, E.; Hiar, A.; et al. Development of a 90-minute integrated noninvasive urinary assay for bladder cancer detection. J. Urol. 2018, 199, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Feber, A.; Dhami, P.; Dong, L.; de Winter, P.; Tan, W.S.; Martínez-Fernández, M.; Paul, D.S.; Hynes-Allen, A.; Rezaee, S.; Gurung, P.; et al. Uromark—a urinary biomarker assay for the detection of bladder cancer. Clin. Epigenet. 2017, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, N.; Mor, Y.; Idelevich, P.; Terkieltaub, D.; Ziv, V.; Elkeles, A.; Lew, S.; Okon, E.; Laufer, M.; Ramon, J.; et al. A novel urine cytology stain for the detection and monitoring of bladder cancer. J. Urol. 2014, 192, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 4 June 2020).
- Lin, Y.H.; Chen, Y.J.; Lai, C.S.; Chen, Y.T.; Chen, C.L.; Yu, J.S.; Chang, Y.S. A negative-pressure-driven microfluidic chip for the rapid detection of a bladder cancer biomarker in urine using bead-based enzyme-linked immunosorbent assay. Biomicrofluidics 2013, 7, 24103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, R.; Soares, J.; Gaiteiro, C.; Peixoto, A.; Lima, L.; Ferreira, D.; Relvas-Santos, M.; Fernandes, E.; Tavares, A.; Cotton, S.; et al. Glycan affinity magnetic nanoplatforms for urinary glycobiomarkers discovery in bladder cancer. Talanta 2018, 184, 347–355. [Google Scholar] [CrossRef]
- Pursey, J.P.; Chen, Y.; Stulz, E.; Park, M.K.; Kongsuphol, P. Microfluidic electrochemical multiplex detection of bladder cancer DNA markers. Sens. Actuat. B: Chem. 2017, 251, 34–39. [Google Scholar] [CrossRef]
- Woo, H.K.; Sunkara, V.; Park, J.; Kim, T.H.; Han, J.R.; Kim, C.J.; Choi, H.I.; Kim, Y.K.; Cho, Y.K. Exodisc for rapid, size-selective, and efficient isolation and analysis of nanoscale extracellular vesicles from biological samples. ACS Nano 2017, 11, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.G.; Kong, M.Q.; Zhou, S.; Sheng, Y.F.; Wang, P.; Yu, T.; Inci, F.; Kuo, W.P.; Li, L.J.; Demirci, U.; et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 2017, 7, 46224. [Google Scholar] [CrossRef]
- Macgregor-Ramiasa, M.; McNicholas, K.; Ostrikov, K.; Li, J.; Michael, M.; Gleadle, J.M.; Vasilev, K. A platform for selective immuno-capture of cancer cells from urine. Biosens. Bioelectron. 2017, 96, 373–380. [Google Scholar] [CrossRef]
- Chuang, C.H.; Du, Y.C.; Wu, T.F.; Chen, C.H.; Lee, D.H.; Chen, S.M.; Huang, T.C.; Wu, H.P.; Shaikh, M.O. Immunosensor for the ultrasensitive and quantitative detection of bladder cancer in point of care testing. Biosens. Bioelectron. 2016, 84, 126–132. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Zanganeh, S.; Akbarnejad, E.; Salehi, F.; Abdolahad, M. Microfluidic device for label-free quantitation and distinction of bladder cancer cells from the blood cells using micro machined silicon based electrical approach; suitable in urinalysis assays. J. Pharm Biomed. Anal. 2017, 134, 36–42. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Ortiz-Bonilla, C.J.; Lee, Y.-F. Extracellular vesicles in bladder cancer: Biomarkers and beyond. Int. J. Mol. Sci. 2018, 19, 2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oeyen, E.; Hoekx, L.; De Wachter, S.; Baldewijns, M.; Ameye, F.; Mertens, I. Bladder cancer diagnosis and follow-up: The current status and possible role of extracellular vesicles. Int. J. Mol. Sci. 2019, 20, 821. [Google Scholar] [CrossRef] [Green Version]
- van Bokhoven, A.; Scott Lucia, M. Requisite for Collection and Distribution of Tissue and Fluid Specimens for Molecular Diagnostics and Discovery in Bladder Cancer. In Precision Molecular Pathology of Bladder Cancer; Hansel, D.E., Lerner, S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- NICE. Bladder cancer: Diagnosis and management of bladder cancer. BJU Int. 2017, 120, 755–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babjuk, M.; Burger, M.; Compérat, E.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Rouprêt, M.; Shariat, S.F.; Sylvester, R.; et al. Eau Guidelines on Non-muscle-invasive Bladder Cancer. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 13 January 2020).
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Nccn Guidelines Version 4.2019 on Bladder Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (accessed on 13 January 2020).
- Georges, J.F.; Valeri, A.; Wang, H.; Brooking, A.; Kakareka, M.; Cho, S.S.; Al-Atrache, Z.; Bamimore, M.; Osman, H.; Ifrach, J.; et al. Delta-aminolevulinic acid-mediated photodiagnoses in surgical oncology: A historical review of clinical trials. Front. Surg. 2019, 6, 45. [Google Scholar] [CrossRef]
- Kriegmair, M.; Baumgartner, R.; Knüchel, R.; Ehsan, A.; Steinbach, P.; Lumper, W.; Hofstädter, F.; Hofstetter, A. Photodynamic diagnosis of urothelial neoplasms after intravesicular instillation of 5-aminolevulinic acid. Urol. A 1994, 33, 270–275. [Google Scholar]
- Karl, A.; Tritschler, S.; Stanislaus, P.; Gratzke, C.; Tilki, D.; Strittmatter, F.; Knuchel, R.; Stief, C.G.; Zaak, D. Positive urine cytology but negative white-light cystoscopy: An indication for fluorescence cystoscopy? BJU Int. 2009, 103, 484–487. [Google Scholar] [CrossRef]
- Drejer, D.; Béji, S.; Oezeke, R.; Nielsen, A.M.; Høyer, S.; Bjerklund Johansen, T.E.; Lam, G.W.; Jensen, J.B. Comparison of white light, photodynamic diagnosis, and narrow-band imaging in detection of carcinoma in situ or flat dysplasia at transurethral resection of the bladder: The dablaca-8 study. Urology 2017, 102, 138–142. [Google Scholar] [CrossRef]
- Inoue, K.; Anai, S.; Fujimoto, K.; Hirao, Y.; Furuse, H.; Kai, F.; Ozono, S.; Hara, T.; Matsuyama, H.; Oyama, M.; et al. Oral 5-aminolevulinic acid mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: A randomized, double-blind, multicentre phase ii/iii study. Photodiagn. Photodyn. Ther. 2015, 12, 193–200. [Google Scholar] [CrossRef]
- McNicholas, K.; MacGregor, M.N.; Gleadle, J.M. In order for the light to shine so brightly, the darkness must be present—why do cancers fluoresce with 5-aminolaevulinic acid? Br. J. Cancer 2019, 121, 631–639. [Google Scholar] [CrossRef]
- Sharwani, A.; Alharbi, F.A. Monitoring of photobleaching in photodynamic therapy using fluorescence spectroscopy. Gulf. J. Oncol. 2014, 1, 79–83. [Google Scholar]
- Nicholls, D. Available online: https://www.naturespot.org.uk/species/perforate-st-johns-wort (accessed on 29 April 2019).
- Skalkos, D.; Gioti, E.; Stalikas, C.D.; Meyer, H.; Papazoglou, T.G.; Filippidis, G. Photophysical properties of hypericum perforatum l. Extracts—novel photosensitizers for pdt. J. Photochem. Photobiol. B: Biol. 2006, 82, 146–151. [Google Scholar] [CrossRef] [PubMed]
- D’Hallewin, M.A.; De Witte, P.A.; Waelkens, E.; Merlevede, W.; Baert, L. Fluorescence detection of flat bladder carcinoma in situ after intravesical instillation of hypericin. J. Urol. 2000, 164, 349–351. [Google Scholar] [CrossRef]
- Vandepitte, J.; Van Cleynenbreugel, B.; Hettinger, K.; Van Poppel, H.; de Witte, P.A.M. Biodistribution of pvp-hypericin and hexaminolevulinate-induced ppix in normal and orthotopic tumor-bearing rat urinary bladder. Cancer Chemother. Pharmacol. 2011, 67, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, P.; Weingandt, H.; Baumgartner, R.; Kriegmair, M.; Hofstadter, F.; Knuchel, R. Cellular fluorescence of the endogenous photosensitizer protoporphyrin ix following exposure to 5-aminolevulinic acid. Photochem. Photobiol. 1995, 62, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, R.; Fuchs, C.; Kriegmair, M. Photodynamic effects of 5-aminolevulinic acid-induced porphyrin on human bladder carcinoma cells in vitro. Eur. J. Cancer 1996, 32, 328–334. [Google Scholar] [CrossRef]
- Li, G.; Szewczuk, M.R.; Raptis, L.; Johnson, J.G.; Weagle, G.E.; Pottier, R.H.; Kennedy, J.C. Rodent fibroblast model for studies of response of malignant cells to exogenous 5-aminolevulinic acid. Br. J. Cancer 1999, 80, 676–684. [Google Scholar] [CrossRef]
- Seidl, J.; Rauch, J.; Krieg, R.C.; Appel, S.; Baumgartner, R.; Knuechel, R. Optimization of differential photodynamic effectiveness between normal and tumor urothelial cells using 5-aminolevulinic acid-induced protoporphyrin ix as sensitizer. Int. J. Cancer 2001, 92, 671–677. [Google Scholar] [CrossRef]
- Pytel, A.; Schmeller, N. New aspect of photodynamic diagnosis of bladder tumors: Fluorescence cytology. Urology 2002, 59, 216–219. [Google Scholar] [CrossRef]
- Tauber, S.; Schneede, P.; Liedl, B.; Liesmann, F.; Zaak, D.; Hofstetter, A. Fluorescence cytology of the urinary bladder. Urology 2003, 61, 1067–1071. [Google Scholar] [CrossRef]
- Tauber, S.; Stepp, H.; Meier, R.; Bone, A.; Hofstetter, A.; Stief, C. Integral spectrophotometric analysis of 5-aminolaevulinic acid-induced fluorescence cytology of the urinary bladder. BJU Int. 2006, 97, 992–996. [Google Scholar] [CrossRef]
- Inoue, K.; Ota, U.; Ishizuka, M.; Kawada, C.; Fukuhara, H.; Shuin, T.; Okura, I.; Tanaka, T.; Ogura, S. Porphyrins as urinary biomarkers for bladder cancer after 5-aminolevulinic acid (ala) administration: The potential of photodynamic screening for tumors. Photodiagn. Photodyn. Ther. 2013, 10, 484–489. [Google Scholar] [CrossRef]
- Miyake, M.; Nakai, Y.; Anai, S.; Tatsumi, Y.; Kuwada, M.; Onishi, S.; Chihara, Y.; Tanaka, N.; Hirao, Y.; Fujimoto, K. Diagnostic approach for cancer cells in urine sediments by 5-aminolevulinic acid-based photodynamic detection in bladder cancer. Cancer Sci. 2014, 105, 616–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, Y.; Anai, S.; Onishi, S.; Masaomi, K.; Tatsumi, Y.; Miyake, M.; Chihara, Y.; Tanaka, N.; Hirao, Y.; Fujimoto, K. Protoporphyrin ix induced by 5-aminolevulinic acid in bladder cancer cells in voided urine can be extracorporeally quantified using a spectrophotometer. Photodiagn. Photodyn. Ther. 2015, 12, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, G.; Nakata, W.; Tani, M.; Tsujimura, G.; Tsujimoto, Y.; Nin, M.; Mimura, A.; Miwa, H.; Tsujihata, M. High diagnostic efficacy of 5-aminolevulinic acid-induced fluorescent urine cytology for urothelial carcinoma. Int. J. Clin. Oncol. 2019, 24, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Čunderlíková, B.; Wahlqvist, R.; Berner, A.; Vasovič, V.; Warloe, T.; Nesland, J.M.; Peng, Q. Detection of urinary bladder cancer with flow cytometry and hexaminolevulinate in urine samples. Cytopathology 2007, 18, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Vasilev, K.; Shirazi, H.S.; McNicholas, K.; Li, J.; Gleadle, J.; Macgregor, M. Biosensor device for the photo-specific detection of immuno-captured bladder cancer cells using hexaminolevulinate: An ex-vivo study. Photodiagn. Photodyn. Ther. 2019, 28, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Anai, S.; Kuwada, M.; Miyake, M.; Chihara, Y.; Tanaka, N.; Hirayama, A.; Yoshida, K.; Hirao, Y.; Fujimoto, K. Photodynamic diagnosis of shed prostate cancer cells in voided urine treated with 5-aminolevulinic acid. BMC Urol. 2014, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Nakai, Y.; Ozawa, T.; Mizuno, F.; Onishi, S.; Owari, T.; Hori, S.; Morizawa, Y.; Tatsumi, Y.; Miyake, M.; Tanaka, N.; et al. Spectrophotometric photodynamic detection involving extracorporeal treatment with hexaminolevulinate for bladder cancer cells in voided urine. J. Cancer Res. Clin. Oncol. 2017, 143, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.Y.; Ng, B.K.; Razul, S.G.; Chin, W.W.; Tan, P.H.; Lau, W.K.; Olivo, M. Fluorescence detection of bladder cancer using urine cytology. Int. J. Oncol. 2007, 31, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Crnolatac, I.; Huygens, A.; van Aerschot, A.; Busson, R.; Rozenski, J.; de Witte, P.A.M. Synthesis, in vitro cellular uptake and photo-induced antiproliferative effects of lipophilic hypericin acid derivatives. Bioorg. Med. Chem. 2005, 13, 6347–6353. [Google Scholar] [CrossRef]
- Olivo M, L.W.; Manivasager, V.; Bhuvaneswari, R.; Wei, Z.; Soo, K.C.; Cheng, C.; Tan, P.H. Novel photodynamic diagnosis of bladder cancer: Ex vivo fluorescence cytology using hypericin. Int. J. Oncol. 2003, 23, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Miyake, M.; Anai, S.; Hori, S.; Tatsumi, Y.; Morizawa, Y.; Onisi, S.; Tanaka, N.; Fujimoto, K. Spectrophotometric photodynamic diagnosis of prostate cancer cells excreted in voided urine using 5-aminolevulinic acid. Lasers Med. Sci. 2018, 33, 1557–1563. [Google Scholar] [CrossRef]
- Krieg, R.C.; Fickweiler, S.; Wolfbeis, O.S.; Knuechel, R. Cell-type specific protoporphyrin ix metabolism in human bladder cancer in vitro. Photochem. Photobiol. 2000, 72, 226–233. [Google Scholar] [CrossRef]
- Olivo, M.; Lau, W.; Manivasager, V.; Hoon, T.P.; Christopher, C. Fluorescence confocal microscopy and image analysis of bladder cancer using 5-aminolevulinic acid. Int. J. Oncol. 2003, 22, 523–528. [Google Scholar] [PubMed]
- Inoue, K.; Karashima, T.; Kamada, M.; Shuin, T.; Kurabayashi, A.; Furihata, M.; Fujita, H.; Utsumi, K.; Sasaki, J. Regulation of 5-aminolevulinic acid-mediated protoporphyrin ix accumulation in human urothelial carcinomas. Pathobiology 2009, 76, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, G.; Nakata, W.; Yoshimura, A.; Tsujimura, G.; Tsujimoto, Y.; Nin, M.; Mimura, A.; Miwa, H.; Tsujihata, M. High performance of 5-aminolevulinic acid-induced fluorescent selective upper tract urinary cytology. Int. J. Urol. 2020, 27, 213–218. [Google Scholar] [CrossRef]
- Shirazi, H.S.; Chan, K.M.; Rouget, J.; Ostrikov, K.; McNicholas, K.; Li, J.; Butler, L.; Gleadle, J.M.; Vasilev, K.; MacGregor, M. Plasma enabled devices for the selective capture and photodynamic identification of prostate cancer cells. Biointerphases 2020, 15, 031002. [Google Scholar] [CrossRef]
- Perotti, C.; Fukuda, H.; DiVenosa, G.; MacRobert, A.J.; Batlle, A.; Casas, A. Porphyrin synthesis from ala derivatives for photodynamic therapy. In vitro and in vivo studies. Br. J. Cancer 2004, 90, 1660–1665. [Google Scholar] [CrossRef] [Green Version]
- Fotinos, N.; Convert, M.; Piffaretti, J.C.; Gurny, R.; Lange, N. Effects on gram-negative and gram-positive bacteria mediated by 5-aminolevulinic acid and 5-aminolevulinic acid derivatives. Antimicrob. Agents Chemother. 2008, 52, 1366–1373. [Google Scholar] [CrossRef] [Green Version]
- Kamuhabwa, A.R.; Agostinis, P.; D’Hallewin, M.A.; Kasran, A.; de Witte, P.A. Photodynamic activity of hypericin in human urinary bladder carcinoma cells. Anticancer Res. 2000, 20, 2579–2584. [Google Scholar]
- Huygens, A.N.N.; Kamuhabwa, A.R.; Roskams, T.; Van Cleynenbreugel, B.E.N.; Van Poppel, H.; de Witte, P.A.M. Permeation of hypericin in spheroids composed of different grade transitional cell carcinoma cell lines and normal human urothelial cells. J. Urol. 2005, 174, 69–72. [Google Scholar] [CrossRef] [PubMed]





| Test (Manufacturer) | Detected Biomarker | Assay type | Sensitivity % | Specificity % | Development Stage * | FDA Approved | Ref. |
|---|---|---|---|---|---|---|---|
| Urine Cytology | Atypical urothelial cells | Microscopy | 33.3 | 100 | Clinical practice | NA | [4] |
| NMP22/BladderChek® (Abbott Laboratories, IL, USA) | Nuclear mitotic apparatus proteins (Nuclear matrix protein-22) | Sandwich ELISA/point-of-care test | 33–77 | 75–97 | FDA approved diagnosis and follow-up | 1996/2002 | [18] |
| uCyt+™/Immunocyt™ (Scimedx Corporation, NJ, USA) | Bladder tumour cell associated mucins/carcinoembryonic antigen (antibodies19A211, LDQ10 and M344) | Immunocytochemistry | 78–90 | 77–87 | FDA approved follow-up | 2000 | [18] |
| UroVysion ™ (Abbott Laboratories, IL, USA) | Aneuploidy and loss of loci (chromosomes 3, 7, 17 and 9p21 loci) | Multicoloured and multiprobed FISH | 50–88 | 87–98 | FDA approved diagnosis and follow-up | 2002 | [18] |
| BTA stat®/TRAK® (Polymedco Inc., NY, USA) | Complement factor H-related protein | Dipstick immunoassay/sandwich ELISA | 61–87 | 38–87 | FDA approved diagnosis and follow-up | 1998/1997 | [18] |
| UBC-Rapid/ELISA test (IDL Biotech AB, Bromma, Sweden) | Cytoskeletal protein (cytokeratin 8 and 18) | Sandwich ELISA/point-of-care test | 48.7–70.5 | 64.5–79.3 | Clinical laboratory research | [4] | |
| CYFRA 21-1 (Roche Diagnostics, IN, USA) | Cytoskeletal protein (cytokeratin 19) | Electrochemiluminescent immunoassay/ELISA/immunoradiometric assay | 82 | 80 | Clinical laboratory research | [25] | |
| BLCA-4 (Eichrom Technologies, IL, USA) | Nuclear matrix protein (BLCA-4) | Sandwich ELISA | 96.4 | 100 | Clinical laboratory research | [26] | |
| Survivin (Fujirebio Diagnostics Inc., PA, USA) | Inhibitor of apoptosis gene | Bio-dot test | 64 | 93 | Clinical trial | [27] | |
| Cx Bladder (Pacific Edge Diagnostics, PA, USA) | mRNA expression of genes (IGF, HOXA, MDK, CDC and IL8R) | RT-qPCR | 91 | 96 | Clinical trial | [28] | |
| AssureMDx (MDxHealth, CA, USA) | Methylation analysis (OTX1, ONECUT2 and TWIST)/mutation analysis (FGFR3, TERT and HRAS) | Methylation/mutation analysis | 57–83 | 59 | Clinical laboratory research | [29] | |
| Xpert® bladder cancer monitor (Cepheid Inc., CA, USA) | mRNA expression of genes (CRH, IGF2, UPK1B, ANXA10 and ABL1) | RT-qPCR | 73 | 77–90 | Clinical trial | [30] | |
| UroMark (Kelly:Feber Lab, UCL, UK) | Targeted loci DNA methylation (150 CpG loci) | Microdroplet-based PCR and NGS | 98 | 97 | Clinical trial | [31] | |
| CellDetect ® (Micromedic Technologies Ltd., Tel Aviv, Israel) | Atypical urothelial cells | Microscopy | 94 | 89 | Clinical trial | [32] |
| Microdevices | Detected Marker | Assay Type | Ref. |
|---|---|---|---|
| Negative pressure-driven microfluidic chip | APOA1 protein via antibody capture on magnetic microbead | ELISA | [34] |
| Magnetic nanoprobes with lectins platform | Glycoproteins via Glycoproteomics and CD44 expression | Slot-blot analysis, immunohistochemistry | [35] |
| Microfluidic multiplex electrochemical sensor | cfDNA via DNA hairpins bound to electrode, DNA methylation | SPR/EIS | [36] |
| Microfluidic antibody capture platform | Cancer cell capture via EpCAM on POx coating | Point-of-care test | [39] |
| Antibody conjugated nanoprobes immunosensor | Intracellular Gal-1 protein via immunosensor | Point-of-care test | [40] |
| Microfluidic label-free silicon-based electrical approach | Whole cells via membrane capacitance difference | Flow cytometry | [41] |
| Microfluidic double filtration | Extracellular vesicles via size filtration | ELISA | [37,38] |
| Study (Year) | Prodrug | Types of Samples Tested | Methodological Approach * | Ref. |
|---|---|---|---|---|
| Steinbach et al. (1995) | 5-ALA | In vitro ● Human urothelial cancer J82 and RT4 cells ● Human normal urothelial HCV29 cells ● Human umbilical cord endothelial cells ● Human fibroblasts | 30–300 µg/mL 5-ALA in serum-containing medium for 4 h at 37 °C | [59] |
| Riesenberg et al. (1996) | 5-ALA | In vitro ● Human urothelial cancer J82 and RT4 cells ● Human normal urothelial HCV29 cells | 25–100 µg/mL 5-ALA in serum-containing RPMI 1640 medium for 4 h at 37 °C | [60] |
| Li et al. (1999) [61] | 5-ALA | In vivo ● Balb/c mice with fibrosarcoma In vitro ● Mouse normal fibroblasts ● Mouse fibrosarcoma cells ● Human bladder carcinoma T24 cells ● Human lung cancer A549 cells | In vivo ●100–300 mg 5-ALA per kg of body weight, injected intraperitoneally for 3 h In vitro ●3mM 5-ALA in RPMI-1640 medium with 1% FCS for 15 h at 37 °C | [61] |
| Krieg et al. (2000) | 5-ALA | In vitro ● Human urothelial cancer J82 and RT4 cells ● Human normal urothelial UROtsa cells ● Human fibroblasts | 10–200 µg/mL 5-ALA in serum-free medium for 3 h at 37 °C | [78] |
| Seidl et al. (2001) | 5-ALA | In vitro ● Human urothelial cancer RT4 cells ● Human normal urothelial UROtsa cells | 100 and 200 µg/mL 5-ALA in serum-free medium for 3 and 1 h at 37 °C | [62] |
| Inoue et al. (2009) | 5-ALA | In vitro ● Human urothelial cancer 253J-P, 253J-BV, T24 cells ● Human prostate carcinoma PC-3 and DU145 cells ● Human renal carcinoma ACHN, 786-o and A-498 cells ● Human renal epithelial RPTEC cells | 0–1 mM 5-ALA in serum-free medium for 0-6 h at 37 °C | [80] |
| Chan et al. (2019) | HAL | Ex vivo ● Trypsinized cells (human bladder cancer HT1197 and HT1376; human fibroblast HFF) in suspension | 10–150 µM HAL in serum-free medium for 30 min to 6 h at 37 °C | [71] |
| Shirazi et al. (2020) | HAL | Ex vivo ● Trypsinized cells (human prostate LNCaP; human normal prostate epithelium PNT2) in suspension | 50–150 µM HAL in PBS for 30 min to 2 h at 4, 23 and 37 °C | [82] |
| Crnolatac (2005) | Hypericin and its derivatives | In vitro ● Human bladder carcinoma RT112 cells | 1 µM hypericin/hypericin derivatives in MEM for 2 h at 37 °C | [75] |
| Study (year) | Prodrug | Cohort Size | Methods | Administration | Specimen | Ref. |
|---|---|---|---|---|---|---|
| Pytel et al. (2002) | 5-ALA | 38 a | 1.5 g 5-ALA in 50 mL of 1.5% sodium bicarbonate for > 1 h | Intravesical instillation | Urine | [63] |
| Tauber et al. (2003) | 5-ALA | 162 a | 50 mL of 3% 5-ALA buffered with sodium monohydrogen-phosphate for 2 h | Intravesical instillation | Lavage solution | [64] |
| Tauber et al. (2006) | 5-ALA | 62 a | 50 mL of 3% 5-ALA buffered with sodium monohydrogen-phosphate for 1 to 2 h | Intravesical instillation | Lavage solution/sediments | [65] |
| Inoue et al. (2013) | 5-ALA | 66 a | 1.0 g 5-ALA | Oral | Urine | [66] |
| Miyake et al. (2014) | 5-ALA | 58 a | 1 mM of 5-ALA in serum-free RPMI-1640 medium for 2 h at 37 °C | Exogenous 5-ALA added to urine pellet | Urine | [67] |
| Nakai et al. (2015) | 5-ALA | 61 a | 1 mM of 5-ALA in PBS for 2 h at 37 °C | Exogenous 5-ALA added to urine pellet | Urine | [68] |
| Nakai et al. (2017) | 5-ALA | 50 a | 1 mM of 5-ALA in PBS for 2 h at 37 °C | Exogenous 5-ALA added to urine pellet | Urine | [73] |
| Yamamichi (2019) | 5-ALA | 160 a | 200 µg/mL 5-ALA in MEM for 2 h at 37 °C | Exogenous 5-ALA added to urine pellet | Urine | [69] |
| Yamamichi (2020) | 5-ALA | 104 a | 1.2 mM 5-ALA in MEM for 2 h at 37 °C | Exogenous 5-ALA added to urine pellet | Urine | [81] |
| Čunderlíková et al. (2007) | HAL | 19 a | 50 µM HAL in serum-free RPMI-1640 medium for 1 h | Exogenous HAL added to urine pellet | Urine | [70] |
| Nakai et al. (2017) | HAL | 50 a | 0.1 mM HAL in PBS for 2 h at 37 °C | Exogenous HAL added to urine pellet | Urine | [73] |
| Nakai et al. (2014) | 5-ALA | 138 b | 1 mM of 5-ALA in PBS for 2 h at 37 °C | Exogenous 5-ALA added to urine pellet | Urine | [72] |
| Nakai et al. (2018) | 5-ALA | 189 b | 1 mM of 5-ALA in PBS for 2 h at 37 °C | Exogenous 5-ALA added to urine pellet | Urine | [77] |
| Pytel et al. (2002) | Hypericin | 8 a | 40 mL of an 8-µmol/L solution of hypericin for > 1 h | Intravesical instillation | Urine | [63] |
| Olivo et al. (2003) | Hypericin | 29 a | 1 mL of hypericin in serum-free RPMI-1640 medium for 15 min at room temperature | Exogenous hypericin added to urine pellet | Urine | [76] |
| Fu et al. (2007) | Hypericin | 21 a | 1 mL of hypericin in serum-free RPMI-1640 medium for 15 min at room temperature | Exogenous hypericin added to urine pellet | Urine | [74] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.M.; Gleadle, J.; Li, J.; Vasilev, K.; MacGregor, M. Shedding Light on Bladder Cancer Diagnosis in Urine. Diagnostics 2020, 10, 383. https://doi.org/10.3390/diagnostics10060383
Chan KM, Gleadle J, Li J, Vasilev K, MacGregor M. Shedding Light on Bladder Cancer Diagnosis in Urine. Diagnostics. 2020; 10(6):383. https://doi.org/10.3390/diagnostics10060383
Chicago/Turabian StyleChan, Kit Man, Jonathan Gleadle, Jordan Li, Krasimir Vasilev, and Melanie MacGregor. 2020. "Shedding Light on Bladder Cancer Diagnosis in Urine" Diagnostics 10, no. 6: 383. https://doi.org/10.3390/diagnostics10060383