Discrimination of Aluminum from Silicon by Electron Crystallography with the JUNGFRAU Detector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Data Acquisition
- Start DM script to record rotation angle alpha
- Start rotation
- Start data capture from JUNGFRAU
- Stop data capture from JUNGFRAU
- Stop rotation
2.3. Data Conversion
2.4. Data Processing
2.5. Structure Solution and Refinement
2.6. Data Availability
| Albite | CSD 2042879 | LTA x1 | CSD 2042880 | LTA x2 | CSD 2042881 |
| LTA x3 | CSD 2042882 | LTA x4 | CSD 2042883 | LTA x5 | CSD 2042884 |
3. Results
3.1. Operation of the JUNGFRAU Detector and Data Conversion
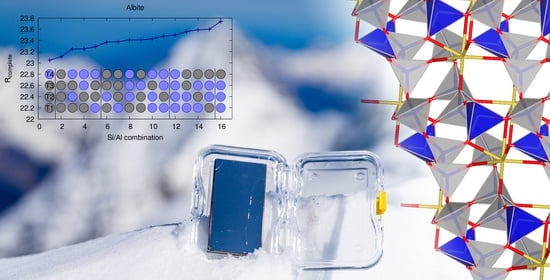
3.2. Benchmark Sample Albite
3.3. Benchmark Sample Zeolite A
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pflugrath, J.W. The finer things in X-ray diffraction data collection. Acta Crystallogr. Sect. 1999, 55, 1718–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förster, A.; Brandstetter, S.; Schulze-Briese, C. Transforming X-ray detection with hybrid photon counting detectors. Philos. Trans. R. Soc. A 2019, 377, 20180241. [Google Scholar] [CrossRef] [PubMed]
- Casanas, A.; Warshamanage, R.; Finke, A.D.; Panepucci, E.; Olieric, V.; Nöll, A.; Tampé, R.; Brandstetter, S.; Förster, A.; Mueller, M.; et al. EIGER detector: Application in macromolecular crystallography. Acta Crystallogr. 2016, D72, 1036–1048. [Google Scholar] [CrossRef]
- Campbell, M. 10 years of the Medipix2 Collaboration. Nucl. Instrum. Methods Phys. Res. Sect. A 2011, 633, S1–S10. [Google Scholar] [CrossRef]
- Tinti, G.; Fröjdh, E.; van Genderen, E.; Gruene, T.; Schmitt, B.; de Winter, D.A.M.; Weckhuysen, B.M.; Abrahams, J.P. Electron Crystallography with the EIGER detector. IUCrJ 2018, 5, 190–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruene, T.; Wennmacher, J.T.C.; Zaubitzer, C.; Holstein, J.J.; Heidler, J.; Fecteau-Lefebvre, A.; De Carlo, S.; Müller, E.; Goldie, K.N.; Regeni, I.; et al. Rapid structure determination of microcrystalline molecular compounds using electron diffraction. Angew. Chem. Int. Ed. 2018, 57, 16313–16317. [Google Scholar] [CrossRef] [Green Version]
- Andrusenko, I.; Hamilton, V.; Mugnaioli, E.; Lanza, A.; Hall, C.; Potticary, J.; Hall, S.R.; Gemmi, M. The Crystal Structure of Orthocetamol Solved by 3D Electron Diffraction. Angew. Chem. Int. Ed. 2019, 58, 10919–10922. [Google Scholar] [CrossRef]
- Simancas, J.; Simancas, R.; Bereciartua, P.J.; Jorda, J.L.; Rey, F.; Corma, A.; Nicolopoulos, S.; Pratim Das, P.; Gemmi, M.; Mugnaioli, E. Ultrafast Electron Diffraction Tomography for Structure Determination of the New Zeolite ITQ-58. J. Am. Chem. Soc. 2016, 138, 10116–10119. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Kapaca, E.; Li, J.; Liu, Y.; Yi, X.; Zheng, A.; Zou, X.; Jiang, J. An Extra-Large-Pore Zeolite with 24 × 8 × 8-Ring Channels Using a Structure-Directing Agent Derived from Traditional Chinese Medicine. Angew. Chem. Int. Ed. 2018, 57, 6486–6490. [Google Scholar] [CrossRef]
- Nangia, A.K.; Desiraju, G.R. Crystal Engineering: An Outlook for the Future. Angew. Chem. Int. Ed. 2019, 58, 4100–4107. [Google Scholar] [CrossRef]
- Kunde, T.; Schmidt, B.M. Microcrystal Electron Diffraction (MicroED) for Small-Molecule Structure Determination. Angew. Chem. Int. Ed. 2019, 58, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Sitsel, O.; Raunser, S. Big insights from tiny crystals. Nat. Chem. 2019, 11, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Mozzanica, A.; Bergamaschi, A.; Brueckner, M.; Cartier, S.; Dinapoli, R.; Greiffenberg, D.; Jungmann-Smith, J.; Maliakal, D.; Mezza, D.; Ramilli, M.; et al. Characterization results of the JUNGFRAU full scale readout ASIC. J. Instrum. 2016, 11, C02047. [Google Scholar] [CrossRef]
- van Schayck, J.P.; van Genderen, E.; Maddox, E.; Roussel, L.; Boulanger, H.; Fröjdh, E.; Abrahams, J.P.; Peters, P.J.; Ravelli, R.B. Sub-pixel electron detection using a convolutional neural network. Ultramicroscopy 2020, 218, 113091. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Fischer, P.; Rohe, T.; Wermes, N. Pixel Detectors: From Fundamentals to Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. 2016. Available online: http://www.iza-structure.org/databases (accessed on 14 December 2020).
- Pluth, J.J.; Smith, J.V. Crystal structure of dehydrated potassium-exchanged Zeolite A. Absence of supposed zero-coordinated potassium. Refinement of silicon, aluminum-ordered superstructure. J. Phys. Chem. 1979, 83, 741–749. [Google Scholar] [CrossRef]
- Pluth, J.J.; Smith, J.V. Accurate redetermination of crystal structure of dehydrated zeolite A. Absence of near zero coordination of sodium. Refinement of silicon, aluminum-ordered superstructure. J. Am. Chem. Soc. 1980, 102, 4704–4708. [Google Scholar] [CrossRef]
- Hayashi, H.; Côté, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Zeolite A imidazolate frameworks. Nat. Mater. 2007, 6, 501–506. [Google Scholar] [CrossRef]
- Jones, J.B. Al–O and Si–O tetrahedral distances in aluminosilicate framework structures. Acta Crystallogr. 1968, B24, 355–358. [Google Scholar] [CrossRef]
- Gemmi, M.; Lanza, A.E. 3D electron diffraction techniques. Acta Crystallogr. 2019, B75, 495–504. [Google Scholar] [CrossRef]
- Williams, T.; Kelley, C. GNUPLOT. 2019. Available online: http://www.gnuplot.info (accessed on 14 December 2020).
- Redford, S.; Andrä, M.; Barten, R.; Bergamaschi, A.; Brückner, M.; Dinapoli, R.; Fröjdh, E.; Greiffenberg, D.; Lopez-Cuenca, C.; Mezza, D.; et al. First, full dynamic range calibration of the JUNGFRAU photon detector. J. Instrum. 2018, 13, C01027. [Google Scholar] [CrossRef]
- Clabbers, M.T.B.; Gruene, T.; Parkhurst, J.M.; Abrahams, J.P.; Waterman, D.G. Electron diffraction data processing with DIALS. Acta Crystallogr. 2018, D74, 506–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karplus, P.A.; Diederichs, K. Linking Crystallographic Model and Data Quality. Science 2012, 336, 1030–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diederichs, K. Quantifying instrument errors in macromolecular X-ray data sets. Acta Crystallogr. 2010, D66, 733–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assmann, G.M.; Wang, M.; Diederichs, K. Making a difference in multi-data-set crystallography: Simple and deterministic data-scaling/selection methods. Acta Crystallogr. 2020, D76, 636–652. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacovazzo, C. (Ed.) Fundamentals of Crystallography; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Wang, B.; Zou, X.; Smeets, S. Automated serial rotation electron diffraction combined with cluster analysis: An efficient multi-crystal workflow for structure determination. IUCrJ 2019, 6, 854–867. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Prince, E. (Ed.) International Tables for Crystallography, 3rd ed.; Wiley: Hoboken, NJ, USA, 2006; Volume C. [Google Scholar]
- Peng, L.M. Electron atomic scattering factors and scattering potentials of crystals. Micron 1999, 30, 625–648. [Google Scholar] [CrossRef]
- Luebben, J.; Gruene, T. New Method to compute Rcomplete enables Maximum Likelihood Refinement for Small Data Sets. Proc. Natl. Acad. Sci. USA 2015, 112, 8999–9003. [Google Scholar] [CrossRef] [Green Version]
- Arvai, A.S. ADXV. A Program to Display X-ray Diffraction Images. 2018. Available online: https://www.scripps.edu/tainer/arvai/adxv.html (accessed on 14 December 2020).
- Heidler, J.; Pantelic, R.; Wennmacher, J.T.C.; Zaubitzer, C.; Fecteau-Lefebvre, A.; Goldie, K.N.; Müller, E.; Holstein, J.J.; van Genderen, E.; De Carlo, S.; et al. Design guidelines for an electron diffractometer for structural chemistry and structural biology. Acta Crystallogr. 2019, D75, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Boscardin, M.; Ceccarelli, R.; Dalla Betta, G.F.; Darbo, G.; Dinardo, M.E.; Giacomini, G.; Menasce, D.; Mendicino, R.; Meschini, M.; Messineo, A.; et al. Performance of new radiation-tolerant thin planar and 3D columnar n+ on p silicon pixel sensors up to a maximum fluence of ≈5 × 1015 neq/cm2. Nucl. Instrum. Methods Phys. Res. Sect. A 2020, 953, 163222. [Google Scholar] [CrossRef]
- Leslie, A.G.W. Integration of macromolecular diffraction data. Acta Crystallogr. 1999, D55, 1696–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, P. Scaling and assessment of data quality. Acta Crystallogr. 2006, D62, 72–82. [Google Scholar] [CrossRef]
- Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. 2010, D66, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Yonekura, K.; Matsuoka, R.; Yamashita, Y.; Yamane, T.; Ikeguchi, M.; Kidera, A.; Maki-Yonekura, S. Ionic scattering factors of atoms that compose biological molecules. IUCrJ 2018, 5, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Gruza, B.; Chodkiewicz, M.L.; Krzeszczakowska, J.; Dominiak, P.M. Refinement of organic crystal structures with multipolar electron scattering factors. Acta Crystallogr. 2020, A76, 92–109. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Petříček, V.; Corrêa, C.A. Structure refinement using precession electron diffraction tomography and dynamical diffraction: Theory and implementation. Acta Crystallogr. 2015, A71, 235–244. [Google Scholar] [CrossRef]
- Brázda, P.; Palatinus, P.; Babor, M. Electron diffraction determines molecular absolute configuration in a pharmaceutical nanocrystal. Science 2019, 364, 667–669. [Google Scholar] [CrossRef]
- Debost, M.; Klar, P.B.; Barrier, N.; Clatworthy, E.B.; Grand, J.; Laine, F.; Brázda, P.; Palatinus, L.; Nesterenko, N.; Boullay, P.; et al. Synthesis of Discrete CHA Zeolite Nanocrystals without Organic Tefor Selective CO2 Capture. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Palatinus, L.; Brázda, P.; Boullay, P.; Perez, O.; Klementová, M.; Petit, S.; Eigner, V.; Zaarour, M.; Mintova, S. Hydrogen positions in single nanocrystals revealed by electron diffraction. Science 2017, 355, 166–169. [Google Scholar] [CrossRef] [PubMed]






| # Al | T1 | T2 | T3 | T4 | R1 | Rcomplete |
|---|---|---|---|---|---|---|
| 0 × Al | Si | Si | Si | Si | 22.80 (17.82) | 23.11 (19.78) |
| 1 × Al | Si | Si | Si | Al | 22.73 (17.76) | 23.05 (19.74) |
| Si | Si | Al | Si | 23.08 (18.11) | 23.41 (20.07) | |
| Si | Al | Si | Si | 23.06 (18.08) | 23.37 (20.12) | |
| Al | Si | Si | Si | 23.05 (18.10) | 23.38 (20.06) | |
| 2 × Al | Si | Si | Al | Al | 22.93 (17.98) | 23.25 (19.95) |
| Si | Al | Si | Al | 22.96 (17.95) | 23.25 (19.97) | |
| Al | Si | Si | Al | 22.95 (17.98) | 23.29 (19.98) | |
| Si | Al | Al | Si | 23.26 (18.29) | 23.60 (20.31) | |
| Al | Si | Al | Si | 23.26 (18.31) | 23.59 (20.26) | |
| Al | Al | Si | Si | 23.24 (18.27) | 23.56 (20.31) | |
| 3 × Al | Si | Al | Al | Al | 23.08 (18.13) | 23.41 (20.14) |
| Al | Si | Al | Al | 23.14 (18.20) | 23.48 (20.16) | |
| Al | Al | Si | Al | 23.10 (18.09) | 23.43 (20.15) | |
| Al | Al | Al | Si | 23.41 (18.42) | 23.74 (20.45) | |
| 4 × Al | Al | Al | Al | Al | 23.18 (18.22) | 23.50 (20.22) |
| Sample | T1 Modelled as/T2 Modelled as | ||||
|---|---|---|---|---|---|
| Si/Al | Si/Si | Al/Al | Al/Si | ||
| x1 (RT) | R1 [%] | 32.62 (31.42) | 32.67 (31.32) | 33.83 (32.51) | 33.95 (32.50) |
| Rcomplete [%] | 33.51 (32.31) | 33.54 (32.19) | 34.94 (33.62) | 34.96 (33.50) | |
| x2 (RT) | R1 [%] | 30.32 (29.75) | 30.66 (30.11) | 31.23 (30.69) | 31.18 (30.55) |
| Rcomplete [%] | 31.18 (30.61) | 31.47 (30.92) | 32.22 (31.68) | 32.07 (31.45) | |
| x3 (−189 °C) | R1 [%] | 27.47 (24.15) | 27.67 (24.19) | 28.01 (24.42) | 28.10 (24.65) |
| Rcomplete [%] | 29.12 (25.82) | 29.30 (25.82) | 29.70 (26.10) | 29.87 (26.43) | |
| x4 (−189 °C) | R1 [%] | 30.80 (28.39) | 30.87 (28.36) | 31.29 (28.61) | 31.26 (28.80) |
| Rcomplete [%] | 31.82 (29.37) | 31.83 (29.31) | 32.35 (29.65) | 32.35 (29.88) | |
| x5 (−189 °C) | R1 [%] | 27.42 (26.76) | 27.57 (26.92) | 27.99 (27.25) | 28.28 (27.65) |
| Rcomplete [%] | 28.30 (27.65) | 28.44 (27.79) | 28.93 (28.19) | 29.25 (28.63) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröjdh, E.; Wennmacher, J.T.C.; Rzepka, P.; Mozzanica, A.; Redford, S.; Schmitt, B.; van Bokhoven, J.A.; Gruene, T. Discrimination of Aluminum from Silicon by Electron Crystallography with the JUNGFRAU Detector. Crystals 2020, 10, 1148. https://doi.org/10.3390/cryst10121148
Fröjdh E, Wennmacher JTC, Rzepka P, Mozzanica A, Redford S, Schmitt B, van Bokhoven JA, Gruene T. Discrimination of Aluminum from Silicon by Electron Crystallography with the JUNGFRAU Detector. Crystals. 2020; 10(12):1148. https://doi.org/10.3390/cryst10121148
Chicago/Turabian StyleFröjdh, Erik, Julian T. C. Wennmacher, Przemyslaw Rzepka, Aldo Mozzanica, Sophie Redford, Bernd Schmitt, Jeroen A. van Bokhoven, and Tim Gruene. 2020. "Discrimination of Aluminum from Silicon by Electron Crystallography with the JUNGFRAU Detector" Crystals 10, no. 12: 1148. https://doi.org/10.3390/cryst10121148