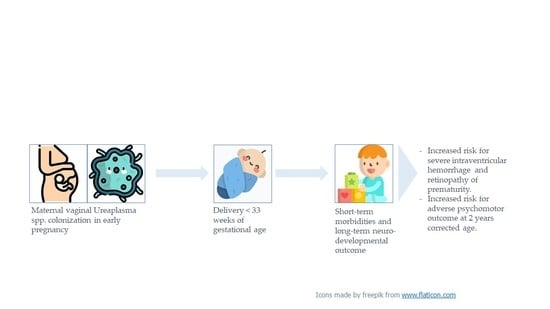
Maternal Vaginal Ureaplasma spp. Colonization in Early Pregnancy Is Associated with Adverse Short- and Long-Term Outcome of Very Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Clinical Outcome
2.3. Neurodevelopmental Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Short-Term Outcome
3.3. Long-Term Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sweeney, E.L.; Dando, S.J.; Kallapur, S.G.; Knox, C.L. The Human Ureaplasma Species as Causative Agents of Chorioam-nionitis. Clin. Microbiol. Rev. 2017, 30, 349–379. [Google Scholar] [CrossRef] [Green Version]
- DiGiulio, D.B.; Romero, R.; Kusanovic, J.P.; Gómez, R.; Kim, C.J.; Seok, K.S.; Gotsch, F.; Mazaki-Tovi, S.; Vaisbuch, E.; Sanders, K.; et al. Prevalence and Diversity of Microbes in the Amniotic Fluid, the Fetal Inflammatory Response, and Pregnancy Outcome in Women with Preterm Pre-Labor Rupture of Membranes. Am. J. Reprod. Immunol. 2010, 64, 38–57. [Google Scholar] [CrossRef] [Green Version]
- Witt, A.; Berger, A.; Gruber, C.J.; Petricevic, L.; Apfalter, P.; Worda, C.; Husslein, P. Increased intrauterine frequency of Ureaplasma urealyticum in women with preterm labor and preterm premature rupture of the membranes and subsequent cesarean delivery. Am. J. Obstet. Gynecol. 2005, 193, 1663–1669. [Google Scholar] [CrossRef]
- Bastek, J.A.; Gómez, L.M.; Elovitz, M.A. The Role of Inflammation and Infection in Preterm Birth. Clin. Perinatol. 2011, 38, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Abele-Horn, M.; Peters, J.; Genzel-Boroviczeny, O.; Wolff, C.; Zimmermann, A.; Gottschling, W. Vaginal Ureaplasma urea-lyticum colonization: Influence on pregnancy outcome and neonatal morbidity. Infection 1997, 25, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, S.; Yamada, T.; Chou, K.; Nishida, R.; Morikawa, M.; Minami, M.; Yamada, H.; Sakuragi, N.; Minakami, H. Association between Preterm Birth and Vaginal Colonization by Mycoplasmas in Early Pregnancy. J. Clin. Microbiol. 2006, 44, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silwedel, C.; Speer, C.P.; Glaser, K. Ureaplasma-associated prenatal, perinatal, and neonatal morbidities. Expert Rev. Clin. Immunol. 2017, 13, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Rittenschober-Böhm, J.; Waldhoer, T.; Schulz, S.M.; Pimpel, B.; Goeral, K.; Kasper, D.C.; Witt, A.; Berger, A. Vaginal Ureaplasma parvum serovars and spontaneous preterm birth. Am. J. Obstet. Gynecol. 2019, 220, 594.e1–594.e9. [Google Scholar] [CrossRef] [PubMed]
- Rittenschober-Böhm, J.; Waldhoer, T.; Schulz, S.M.; Stihsen, B.; Pimpel, B.; Goeral, K.; Hafner, E.; Sliutz, G.; Kasper, D.C.; Witt, A.; et al. First Trimester Vaginal Ureaplasma Biovar Colonization and Preterm Birth: Results of a Prospective Multicenter Study. Neonatology 2018, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Horie, K.; Yada, Y.; Kono, Y.; Hirashima, C.; Usui, R.; Matsubara, S.; Ohkuchi, A. Vaginal Ureaplasma species increase chorioamnionitis in very preterm infants with preterm premature rupture of the membranes at <28 weeks of gestation. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Kasper, D.C.; Mechtler, T.P.; Böhm, J.; Petricevic, L.; Gleiss, A.; Spergser, J.; Witt, A.; Herkner, K.R.; Berger, A. In utero exposure to Ureaplasma spp. is associated with increased rate of bronchopulmonary dysplasia and intraventricular hemorrhage in preterm infants. J. Périnat. Med. 2011, 39, 331–336. [Google Scholar] [CrossRef]
- Resch, B.; Gutmann, C.; Reiterer, F.; Luxner, J.; Urlesberger, B. Neonatal Ureaplasma urealyticum colonization increases pulmonary and cerebral morbidity despite treatment with macrolide antibiotics. Infection 2015, 44, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Witt, A.; Haiden, N.; Kaider, A.; Klebermasz, K.; Fuiko, R.; Langgartner, M.; Pollak, A. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J. Périnat. Med. 2008, 37, 72–78. [Google Scholar] [CrossRef]
- Ozdemır, R.; Sarı, F.N.; Tunay, Z.O.; Erdeve, O.; Canpolat, F.E.; Oguz, S.S.; Uras, N.; Dılmen, U. The association between res-piratory tract Ureaplasma urealyticum colonization and severe retinopathy of prematurity in preterm infants ≤1250 g. Eye 2012, 26, 992–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viscardi, R.M.; Hashmi, N.; Gross, G.W.; Sun, C.-C.J.; Rodriguez, A.; Fairchild, K.D. Incidence of invasive Ureaplasma in VLBW infants: Relationship to severe intraventricular hemorrhage. J. Perinatol. 2008, 28, 759–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kafetzis, D.A.; Skevaki, C.L.; Skouteri, V.; Gavrili, S.; Peppa, K.; Kostalos, C.; Petrochilou, V.; Michalas, S. Maternal Genital Colonization with Ureaplasma urealyticum Promotes Preterm Delivery: Association of the Respiratory Colonization of Premature Infants with Chronic Lung Disease and Increased Mortality. Clin. Infect. Dis. 2004, 39, 1113–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallard, K.; Schopfer, K.; Bodmer, T. Development of real-time PCR for the differential detection and quantification of Ureaplasma urealyticum and Ureaplasma parvum. J. Microbiol. Methods 2005, 60, 13–19. [Google Scholar] [CrossRef]
- Papile, L.-A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Perlman, J.M.; Risser, R.; Broyles, R.S. Bilateral cystic periventricular leukomalacia in the premature infant: Associated risk factors. Pediatrics 1996, 97, 822–827. [Google Scholar]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [CrossRef]
- Bayley, N. Bayley Scales of Infant Development, 2nd ed.; The Psychological Coprporation: San Antonio, TX, USA, 1993. [Google Scholar]
- Bayley, N. Bayley Scales of Infant Development, 3rd ed.; Deutsche, F., Reuner, G., Rosenkranz, J., Eds.; Pearson: Frankfurt, Germany, 2006. [Google Scholar]
- Fuiko, R.; Oberleitner-Leeb, C.; Klebermass-Schrehof, K.; Berger, A.; Brandstetter, S.; Giordano, V. The Impact of Norms on the Outcome of Children Born Very-Preterm when Using the Bayley-III: Differences between US and German Norms. Neonatology 2019, 116, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Romero, R.; Gervasi, M.T.; Kim, J.S.; Yoo, W.; Lee, D.C.; Mittal, P.; Erez, O.; Kusanovic, J.P.; Hassan, S.S.; et al. Wide-spread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Investig. 2009, 89, 924–936. [Google Scholar] [CrossRef]
- Cassell, G.H.; Davis, R.O.; Waites, K.B.; Brown, M.B.; Marriott, P.A.; Stagno, S.; Davis, J.K. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: Potential effect on outcome of pregnancy. Sex. Transm. Dis. 1983, 10, 294–302. [Google Scholar]
- Gray, D.J.; Robinson, H.B.; Malone, J.; Thomson, R.B. Adverse Outcome in Pregnancy Following Amniotic Fluid Isolation of Ureaplasma urealyticum. Obstet. Gynecol. Surv. 1993, 48, 451–452. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Park, J.S.; Chang, J.W.; Kim, Y.A.; Kim, J.C.; Kim, K.S. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am. J. Obstet. Gynecol. 1998, 179, 1254–1260. [Google Scholar] [CrossRef]
- Olomu, I.N.; Hecht, J.L.; Onderdonk, A.O.; Allred, E.N.; Leviton, A. Extremely Low Gestational Age Newborn Study Investigators Perinatal Correlates of Ureaplasma urealyticum in Placenta Parenchyma of Singleton Pregnancies That End Before 28 Weeks of Gestation. Pediatrics 2009, 123, 1329–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motomura, K.; Romero, R.; Xu, Y.; Theis, K.R.; Galaz, J.; Winters, A.D.; Slutsky, R.; Garcia-Flores, V.; Zou, C.; Levenson, D.; et al. Intra-Amniotic Infection with Ureaplasma parvum Causes Preterm Birth and Neonatal Mortality That Are Prevented by Treatment with Clarithromycin. mBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Schelonka, R.L.; Xiao, L.; Grigsby, P.L.; Novy, M.J. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin. Fetal Neonatal Med. 2009, 14, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.; Romero, R.; Ghezzi, F.; Yoon, B.H.; Mazor, M.; Berry, S.M. The fetal inflammatory response syndrome. Am. J. Obstet. Gynecol. 1998, 179, 194–202. [Google Scholar] [CrossRef]
- Viscardi, R.M.; Muhumuza, C.K.; Rodriguez, A.; Fairchild, K.D.; Sun, C.-C.J.; Gross, G.W.; Campbell, A.B.; Wilson, P.D.; Hester, L.; Hasday, J.D. Inflammatory Markers in Intrauterine and Fetal Blood and Cerebrospinal Fluid Compartments Are Associated with Adverse Pulmonary and Neurologic Outcomes in Preterm Infants. Pediatr. Res. 2004, 55, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Meng, J.; Choonara, I.; Xiong, T.; Wang, Y.; Wang, H.; Qu, Y.; Mu, D. Antenatal infection and intraventricular hemorrhage in preterm infants: A meta-analysis. Medicine 2019, 98, e16665. [Google Scholar] [CrossRef] [PubMed]
- Manimtim, W.M.; Hasday, J.D.; Hester, L.; Fairchild, K.D.; Lovchik, J.C.; Viscardi, R.M. Ureaplasma urealyticum Modulates Endotoxin-Induced Cytokine Release by Human Monocytes Derived from Preterm and Term Newborns and Adults. Infect. Immun. 2001, 69, 3906–3915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaser, K.; Silwedel, C.; Fehrholz, M.; Waaga-Gasser, A.M.; Henrich, B.; Claus, H.; Speer, C.P. Ureaplasma Species Differentially Modulate Pro- and Anti-Inflammatory Cytokine Responses in Newborn and Adult Human Monocytes Pushing the State Toward Pro-Inflammation. Front. Cell. Infect. Microbiol. 2017, 7, 484. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Brauner, A.; Jensen, J.S.; Tullus, K. Induction of human macrophage vascular endothelial growth factor and in-tercellular adhesion molecule-1 by Ureaplasma urealyticum and downregulation by steroids. Biol. Neonate 2002, 82, 22–28. [Google Scholar] [CrossRef]
- Glaser, K.; Speer, C.P. Neonatal CNS infection and inflammation caused by Ureaplasma species: Rare or relevant? Expert Rev. Anti. Infect. Ther. 2015, 13, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, M.A.; Liu, Z.; Wang, X.; Kroenke, C.D.; Houser, L.A.; Dozier, B.L.; Martin, L.D.; Waites, K.B.; McEvoy, C.; Schelonka, R.L.; et al. Beyond the uterine environment: Nonhuman primate model to investigate maternal-fetal and neonatal outcomes following chronic intrauterine infection. Pediatr. Res. 2017, 82, 244–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Normann, E.; Lacaze-Masmonteil, T.; Eaton, F.; Schwendimann, L.; Gressens, P.; Thébaud, B. A novel mouse model of Ureaplasma-induced perinatal inflammation: Effects on lung and brain injury. Pediatr. Res. 2009, 65, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Viscardi, R.M.; Kallapur, S.G. Role of Ureaplasma Respiratory Tract Colonization in Bronchopulmonary Dysplasia Pathogenesis: Current Concepts and Update. Clin. Perinatol. 2015, 42, 719–738. [Google Scholar] [CrossRef] [Green Version]

| Whole Cohort n = 222 | Ureaplasma spp. Negative n = 116 | Ureaplasma spp. Positive n = 106 | p-Value | |
|---|---|---|---|---|
| Birthweight, g mean (SD) | 1264 (432) | 1302 (400) | 1222 (461) | 0.17 |
| Gestational age, weeks mean (SD) | 29.4 (2.6) | 29.6 (2.4) | 29.1 (2.8) | 0.23 |
| Male sex, % | 55.4 | 59.5 | 50.9 | 0.23 |
| Antenatal steroids any, % | 93.2 | 91.2 | 95.2 | 0.29 |
| pPROM, % | 40.1 | 38.8 | 41.5 | 0.68 |
| Singleton pregnancy, % | 36.9 | 31.0 | 43.4 | 0.06 |
| Whole Cohort n = 222 | Ureaplasma spp. Negative n = 116 | Ureaplasma spp. Positive n = 106 | p-Value | |
|---|---|---|---|---|
| EOS, n (%) | 4 (1.8) | 2 (1.7) | 2 (1.8) | 0.99 |
| Any IVH, n (%) | 38 (17.1) | 18 (15.5) | 20 (18.9) | 0.59 |
| Severe IVH, n (%) | 14 (6.3) | 3 (2.6) | 11 (10.4) | 0.03 |
| PVL, n (%) | 6 (2.7) | 2 (1.7) | 4 (3.8) | 0.43 |
| NEC, n (%) | 13 (5.9) | 4 (3.4) | 9 (8.5) | 0.15 |
| Any ROP, n (%) | 35 (15.8) | 12 (10.3) | 23 (21.7) | 0.03 |
| Severe ROP, n (%) | 9 (4.1) | 3 (2.6) | 6 (5.7) | 0.32 |
| BPD 28 days #, n (%) | 59 (26.6) | 27 (23.3) | 32 (30.2) | 0.29 |
| BPD 36 weeks #, n (%) | 17 (7.7) | 10 (8.6) | 7 (6.6) | 0.62 |
| Death, n (%) | 16 (7.2) | 5 (4.3) | 11 (10.4) | 0.12 |
| Ureaplasma spp. Negative n = 55 | Ureaplasma spp. Positive n = 37 | OR (CI), p-Value | |
|---|---|---|---|
| Motor outcome | |||
| Normal (≥85), n (%) | 38 (69.1) | 26 (70.3) | |
| Mildly abnormal (70–84), n (%) | 16 (29.1) | 2 (5.4) | 0.18 (0.04,0.86) p = 0.02 |
| Severely abnormal (<70), n (%) | 1 (1.8) | 9 (24.3) | 13.15 (1.57,110.18) p = 0.005 |
| Mental outcome | |||
| Normal (≥85), n (%) | 29 (52.7) | 16 (43.2) | |
| Mildly abnormal (70–84), n (%) | 14 (25.5) | 9 (24.3) | 1.17 (0.41,3.28) p = 0.8 |
| Severely abnormal (<70), n (%) | 12 (21.8) | 12 (32.4) | 1.81 (0.66,4.96) p = 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rittenschober-Böhm, J.; Habermüller, T.; Waldhoer, T.; Fuiko, R.; Schulz, S.M.; Pimpel, B.; Goeral, K.; Witt, A.; Berger, A.; Pichler, K. Maternal Vaginal Ureaplasma spp. Colonization in Early Pregnancy Is Associated with Adverse Short- and Long-Term Outcome of Very Preterm Infants. Children 2021, 8, 276. https://doi.org/10.3390/children8040276
Rittenschober-Böhm J, Habermüller T, Waldhoer T, Fuiko R, Schulz SM, Pimpel B, Goeral K, Witt A, Berger A, Pichler K. Maternal Vaginal Ureaplasma spp. Colonization in Early Pregnancy Is Associated with Adverse Short- and Long-Term Outcome of Very Preterm Infants. Children. 2021; 8(4):276. https://doi.org/10.3390/children8040276
Chicago/Turabian StyleRittenschober-Böhm, Judith, Tanja Habermüller, Thomas Waldhoer, Renate Fuiko, Stefan M. Schulz, Birgit Pimpel, Katharina Goeral, Armin Witt, Angelika Berger, and Karin Pichler. 2021. "Maternal Vaginal Ureaplasma spp. Colonization in Early Pregnancy Is Associated with Adverse Short- and Long-Term Outcome of Very Preterm Infants" Children 8, no. 4: 276. https://doi.org/10.3390/children8040276