Reduced Graphene Oxide–P25 Nanocomposites as Efficient Photocatalysts for Degradation of Bisphenol A in Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of rGO–P25
2.2. The Catalytic Properties of rGO–P25 under Ultraviolet Light
2.3. rGO–P25 Photocatalyst Degrades Bisphenol A under Visible Light
2.3.1. The Most Efficient Photocatalyst
2.3.2. Effect of Catalyst Concentration
2.3.3. Effect of Solution pH
2.4. Degradation of Bisphenol A under Different Light Sources of the rGO–P25 Photocatalyst
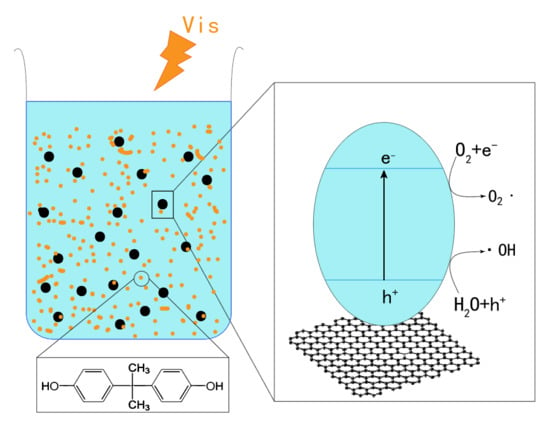
2.5. Enhancement Mechanism of rGO–P25 Photocatalytic Activity
3. Materials and Methods
3.1. Materials, Reagents, and Instruments
3.2. Method for Preparing Graphene
3.3. Synthesis of the rGO Decorated P25 Microspheres
3.4. Characterization
3.5. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zerjav, G.; Djinovic, P.; Pintar, A. TiO2-Bi2O3/(BiO)(2)CO3-reduced graphene oxide composite as an effective visible light photocatalyst for degradation of aqueous bisphenol A solutions. Catal. Today 2018, 315, 237–246. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.-Z.; Li, X.; Zhang, S.-H.; Dai, L.-T.; Liu, X.-Y.; Zhao, X.; Chen, D.-Y.; Feng, X.-Z. Impact of low-dose chronic exposure to Bisphenol A (BPA) on adult male zebrafish adaption to the environmental complexity: Disturbing the color preference patterns and reliving the anxiety behavior. Chemosphere 2017, 186, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, W.Q.; An, W.J.; Liu, L.; Liang, Y.H.; Zhu, Y.F. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Ran, P.; Jiang, L.; Li, X.; Zuo, P.; Li, B.; Li, X.J.; Cheng, X.Y.; Zhang, J.T.; Lu, Y.F. Redox shuttle enhances nonthermal femtosecond two-photon self-doping of rGO-TiO2-x photocatalysts under visible light. J. Mater. Chem. A 2018, 6, 16430–16438. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, B.K. Rapid photo-degradation of 2-chlorophenol under visible light irradiation using cobalt oxide-loaded TiO2/reduced graphene oxide nanocomposite from aqueous media. J. Environ. Manag. 2016, 165, 1–10. [Google Scholar] [CrossRef]
- Zerjav, G.; Arshad, M.S.; Djinovic, P.; Junkar, I.; Kovac, J.; Zavasnik, J.; Pintar, A. Improved electron-hole separation and migration in anatase TiO2 nanorod/reduced graphene oxide composites and their influence on photocatalytic performance. Nanoscale 2017, 9, 4578–4592. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.; Jiang, H.; Jiang, W.; Su, R.; Luo, S.; Luo, Y. Photoelectrocatalytic oxidation of bisphenol A over mesh of TiO2/graphene/Cu2O. Appl. Catal. B Environ. 2016, 183, 75–85. [Google Scholar] [CrossRef]
- Cruz-Ortiz, B.R.; Hamilton, J.W.J.; Pablos, C.; Diaz-Jimenez, L.; Cortes-Hernandez, D.A.; Sharma, P.K.; Castro-Alferez, M.; Fernandez-Ibanez, P.; Dunlop, P.S.M.; Byrne, J.A. Mechanism of photocatalytic disinfection using titania-graphene composites under UV and visible irradiation. Chem. Eng. J. 2017, 316, 179–186. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ismail, A.A.; Kadi, M.W.; Bahnemann, D.W. A comparative study on mesoporous and commercial TiO2 photocatalysts for photodegradation of organic pollutants. J. Photochem. Photobiol. A Chem. 2018, 367, 66–73. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Cruz, M.; Gomez, C.; Duran-Valle, C.J.; Pastrana-Martinez, L.M.; Faria, J.L.; Silva, A.M.T.; Faraldos, M.; Bahamonde, A. Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradationof selected pesticides and influence of the water matrix. Appl. Surf. Sci. 2017, 416, 1013–1021. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, S.; Quan, X.; Yu, H.; Zhao, H. Integration of microfiltration and visible-light-driven photocatalysis on g-C3N4 nanosheet/reduced graphene oxide membrane for enhanced water treatment. Appl. Catal. B Environ. 2016, 194, 134–140. [Google Scholar] [CrossRef]
- Pu, S.Y.; Zhu, R.X.; Ma, H.; Deng, D.L.; Pei, X.J.; Qi, F.; Chu, W. Facile in-situ design strategy todisperse TiO2 nanoparticles on graphene for the enhanced photocatalytic degradation of rhodamine 6G. Appl. Catal. B Environ. 2017, 218, 208–219. [Google Scholar] [CrossRef]
- Lai, C.; Wang, M.M.; Zeng, G.M.; Liu, Y.G.; Huang, D.L.; Zhang, C.; Wang, R.Z.; Xu, P.; Cheng, M.; Huang, C.; et al. Synthesis of surface molecular imprinted TiO2/graphene photocatalyst and its highly efficient photocatalytic degradation of target pollutant under visible light irradiation. Appl. Surf. Sci. 2016, 390, 368–376. [Google Scholar] [CrossRef]
- Wang, S.; Li, D.; Sun, C.; Yang, S.; Guan, Y.; He, H. Synthesis and characterization of g-C3N4/Ag3VO4 composites with significantly enhanced visible-light photocatalytic activity for triphenylmethane dye degradation. Appl. Catal. B Environ. 2014, 144, 885–892. [Google Scholar] [CrossRef]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Ferraro, S.; Seghetti, C.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Enhancement of visible-light photoactivity by polypropylene coated plasmonic Au/TiO2 for dye degradation in water solution. Appl. Surf. Sci. 2018, 441, 575–587. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Xu, P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem. Eng. J. 2017, 310, 389–398. [Google Scholar] [CrossRef]
- Qi, K.Z.; Cheng, B.; Yu, J.G.; Ho, W.K. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Doong, R.A.; Liao, C.Y. Enhanced visible-light-responsive photodegradation of bisphenol A by Cu, N-codoped titanate nanotubes prepared by microwave-assisted hydrothermal method. J. Hazard. Mater. 2017, 322, 254–262. [Google Scholar] [CrossRef] [PubMed]
- He, X.J.; Aker, W.G.; Pelaez, M.; Lin, Y.F.; Dionysiou, D.D.; Hwang, H.M. Assessment of nitrogen-fluorine-codoped TiO2 under visible light for degradation of BPA: Implication for field remediation. J. Photochem. Photobiol. A Chem. 2016, 314, 81–92. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Zeng, J.; Zhang, F.; Zhou, K.; Bowen, C.R.; Zhang, D. Aligned macroporous TiO2/chitosan/reduced graphene oxide (rGO) composites for photocatalytic applications. Appl. Surf. Sci. 2017, 424, 170–176. [Google Scholar] [CrossRef]
- Faraldos, M.; Bahamonde, A. Environmental applications of titania-graphene photocatalysts. Catal. Today 2017, 285, 13–28. [Google Scholar] [CrossRef]
- Wan, J.; Wei, M.; Hu, Z.; Peng, Z.; Wang, B.; Feng, D.; Shen, Y. Ternary composites of TiO2 nanotubes with reduced graphene oxide (rGO) and meso-tetra (4-carboxyphenyl) porphyrin for enhanced visible light photocatalysis. Int. J. Hydrog. Energy 2016, 41, 14692–14703. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hu, R. Synthesis of three-dimensional hierarchically porous reduced graphene oxide–TiO2 nanocomposite for enhanced hydrogen storage. Ceram. Int. 2018, 44, 12458–12465. [Google Scholar] [CrossRef]
- Szabó, T.; Veres, Á.; Cho, E.; Khim, J.; Varga, N.; Dékány, I. Photocatalyst separation from aqueous dispersion using graphene oxide/TiO2 nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 230–239. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.H.; Chen, L.N.; Li, N.; Dong, C.L.; Chen, Q.; Liu, B.B.; Ai, Q.; Si, P.C.; Feng, J.K.; et al. A large-area free-standing graphene oxide multilayer membrane with high stability for nanofiltration applications. Chem. Eng. J. 2018, 345, 536–544. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Y.; Wang, L.; Hu, C. AgBr-wrapped Ag chelated on nitrogen-doped reduced graphene oxide for water purification under visible light. Appl. Catal. B Environ. 2018, 220, 118–125. [Google Scholar] [CrossRef]
- Jiang, G.D.; Lin, Z.F.; Chen, C.; Zhu, L.H.; Chang, Q.; Wang, N.; Wei, W.; Tang, H.Q. TiO2 nanoparticles assembled on graphene oxide nanosheets with high photocatalytic activity for removal of pollutants. Carbon 2011, 49, 2693–2701. [Google Scholar] [CrossRef]
- Wu, H.; Lu, W.; Shao, J.-J.; Zhang, C.; Wu, M.-B.; Li, B.-H.; Yang, Q.-H. pH-dependent size, surface chemistry and electrochemical properties of graphene oxide. New Carbon Mater. 2013, 28, 327–335. [Google Scholar] [CrossRef]
- Bi, H.C.; Xie, X.; Yin, K.B.; Zhou, Y.L.; Wan, S.; He, L.B.; Xu, F.; Banhart, F.; Sun, L.T.; Ruoff, R.S. Spongy Graphene as a Highly Efficient and Recyclable Sorbent for Oils and Organic Solvents. Adv. Funct. Mater. 2012, 22, 4421–4425. [Google Scholar] [CrossRef]
- Yu, X.Q.; Lin, D.M.; Li, P.; Su, Z.Q. Recent advances in the synthesis and energy applications of TiO2-graphene nanohybrids. Sol. Energy Mater. Sol. Cells 2017, 172, 252–269. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Wang, X.; Yu, H.; Yu, J.; Lei, M.; Wang, Y. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B Environ. 2013, 132, 452–459. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Effects of Surface Features on Adsorption of SO2 on Graphite Oxide/Zr(OH)(4) Composites. J. Phys. Chem. C 2010, 114, 14552–14560. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Guo, S.J.; Wang, P.; Xing, L.; Fang, Y.X.; Zhai, Y.M.; Dong, S.J. One-pot, water-phase approach to high-quality graphene/TiO2 composite nanosheets. Chem. Commun. 2010, 46, 7148–7150. [Google Scholar] [CrossRef] [PubMed]
- Han, W.J.; Ren, L.; Gong, L.J.; Qi, X.; Liu, Y.D.; Yang, L.W.; Wei, X.L.; Zhong, J.X. Self-Assembled Three-Dimensional Graphene-Based Aerogel with Embedded Multifarious Functional Nanoparticles and Its Excellent Photoelectrochemical Activities. ACS Sustain. Chem. Eng. 2014, 2, 741–748. [Google Scholar] [CrossRef]
- Simsek, E.B.; Kilic, B.; Asgin, M.; Akan, A. Graphene oxide based heterojunction TiO2-ZnO catalysts with outstanding photocatalytic performance for bisphenol-A, ibuprofen and flurbiprofen. J. Ind. Eng. Chem. 2018, 59, 115–126. [Google Scholar] [CrossRef]
- Liao, G.; Zhu, D.; Zheng, J.; Yin, J.; Lan, B.; Li, L. Efficient mineralization of bisphenol A by photocatalytic ozonation with TiO2-graphene hybrid. J. Taiwan Inst. Chem. Eng. 2016, 67, 300–305. [Google Scholar] [CrossRef]
- Jiang, B.J.; Tian, C.G.; Zhou, W.; Wang, J.Q.; Xie, Y.; Pan, Q.J.; Ren, Z.Y.; Dong, Y.Z.; Fu, D.; Han, J.L.; et al. In Situ Growth of TiO2 in Interlayers of Expanded Graphite for the Fabrication of TiO2-Graphene with Enhanced Photocatalytic Activity. Chem. A Eur. J. 2011, 17, 8379–8387. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.Y.; Zhao, Q.D.; Liu, B.J.; Liu, S.M.; Wang, S.B. Upconversion carbon quantum dots as visible light responsive component for efficient enhancement of photocatalytic performance. J. Colloid Interface Sci. 2017, 496, 425–433. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.L.; Shi, Y.X.; Xu, G.L.; Zhang, E.P.; Wang, H.B.; Kong, Z.; Xi, J.H.; Ji, Z.G. Anatase TiO2 nanosheets with coexposed {101} and {001} facets coupled with ultrathin SnS2 nanosheets as a face-to-face n-p-n dual heterojunction photocatalyst for enhancing photocatalytic activity. Appl. Surf. Sci. 2017, 420, 839–848. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S.; Grygar, T.M.; Bludská, J.; Kormunda, M. TiO2-graphene oxide nanocomposite as advanced photocatalytic materials. Chem. Cent. J. 2013, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.; Jin, P. Photocatalytic activity and mechanism of bisphenol a removal over TiO2-x/rGO nanocomposite driven by visible light. Chem. Eng. J. 2018, 350, 1043–1055. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhao, X.S. Nitrogen-Doped Titanate-Anatase Core–Shell Nanobelts with Exposed {101} Anatase Facets and Enhanced Visible Light Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 5754–5757. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, L.; Zhu, Y. Decontamination of Bisphenol A from Aqueous Solution by Graphene Adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cai, J.; Ding, Z.; Li, Z. TiO2/RGO composite aerogels with controllable and continuously tunable surface wettability for varied aqueous photocatalysis. Appl. Catal. B Environ. 2015, 174–175, 421–426. [Google Scholar] [CrossRef]
- Ali, M.H.H.; Al-Afify, A.D.; Goher, M.E. Preparation and characterization of graphene—TiO2 nanocomposite for enhanced photodegradation of Rhodamine-B dye. Egypt. J. Aquat. Res. 2018, 44, 263–270. [Google Scholar] [CrossRef]
- Rommozzi, E.; Zannotti, M.; Giovannetti, R.; D‘Amato, C.A.; Ferraro, S.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Reduced Graphene Oxide/TiO2 Nanocomposite: From Synthesis to Characterization for Efficient Visible Light Photocatalytic Applications. Catalysts 2018, 8, 598. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z. Comparing Graphene-TiO2 Nanowire and Graphene-TiO2 Nanoparticle Composite Photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 3944–3950. [Google Scholar] [CrossRef]
- Khalid, N.R.; Ahmed, E.; Hong, Z.L.; Ahmad, M. Synthesis and photocatalytic properties of visible light responsive La/TiO2-graphene composites. Appl. Surf. Sci. 2012, 263, 254–259. [Google Scholar] [CrossRef]
- Wang, P.F.; Ao, Y.H.; Wang, C.; Hou, J.; Qian, J. Enhanced photoelectrocatalytic activity for dye degradation by graphene-titania composite film electrodes. J. Hazard. Mater. 2012, 223, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, H.P.; Cui, X.L.; Lin, Y.H. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 2010, 20, 2801–2806. [Google Scholar] [CrossRef]
- Pu, S.H.; Long, D.B.; Liu, Z.H.; Yang, F.Y.; Zhu, J.M. Preparation of RGO-P25 Nanocomposites for the Photocatalytic Degradation of Ammonia in Livestock Farms. Catalysts 2018, 8, 189. [Google Scholar] [CrossRef]
- Liu, L.; Luo, C.; Xiong, J.; Yang, Z.X.; Zhang, Y.B.; Cai, Y.X.; Gu, H.S. Reduced graphene oxide (rGO) decorated TiO2 microspheres for visible-light photocatalytic reduction of Cr(VI). J. Alloy. Compd. 2017, 690, 771–776. [Google Scholar] [CrossRef]
- Bora, L.V.; Mewada, R.K. Visible/solar light active photocatalysts for organic effluent treatment: Fundamentals, mechanisms and parametric review. Renew. Sustain. Energy Rev. 2017, 76, 1393–1421. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Liu, S.; Mathe, V.L. Sonochemical synthesis of CZTS photocatalyst for photocatalytic degradation of phthalic acid. Ultrason. Sonochem. 2019, 56, 284–289. [Google Scholar] [CrossRef]
- Ramasami, A.K.; Reddy, M.V.; Balakrishna, G.R. Combustion synthesis and characterization of NiO nanoparticles. Mater. Sci. Semicond. Process. 2015, 40, 194–202. [Google Scholar] [CrossRef]
- Wang, M.L.; Zhang, M.J.; Zhang, M.X.; Aizezi, M.; Zhang, Y.M.; Hu, J.T.; Wu, G.Z. In-situ mineralized robust polysiloxane-Ag@ZnO on cotton for enhanced photocatalytic and antibacterial activities. Carbohydr. Polym. 2019, 217, 15–25. [Google Scholar] [CrossRef]
- Khatri, A.; Rana, P.S. Visible light photocatalysis of methylene blue using cobalt substituted cubic NiO nanoparticles. Bull. Mater. Sci. 2019, 42, 141. [Google Scholar] [CrossRef] [Green Version]
- Shang, H.Y.; Ma, M.; Liu, F.S.; Miao, Z.; Zhang, A.P. Self-Assembled Reduced Graphene Oxide-TiO2 Thin Film for the Enhanced Photocatalytic Reduction of Cr(VI) Under Simulated Solar Irradiation. J. Nanosci. Nanotechnol. 2019, 19, 3376–3387. [Google Scholar] [CrossRef]
- Shao, L.; Liu, H.; Zeng, W.P.; Zhou, C.Y.; Li, D.; Wang, L.; Lan, Y.Q.; Xu, F.G.; Liu, G.S. Immobilized and photocatalytic performances of PDMS-SiO2-chitosan@ TiO2 composites on pumice under simulated sunlight irradiation. Appl. Surf. Sci. 2019, 478, 1017–1026. [Google Scholar] [CrossRef]
- Teng, W.; Wang, Y.M.; Lin, Q.; Zhu, H.; Tang, Y.B.; Li, X.Y. Synthesis of MoS2/TiO2 Nanophotocatalyst and Its Enhanced Visible Light Driven Photocatalytic Performance. J. Nanosci. Nanotechnol. 2019, 19, 3519–3527. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.; Abbas, A.; Chang, L.C.; Iqbal, A.; Haq, I.U.; Rehman, A.; Awan, S.U.; Arshad, M.; Rafique, M.; Barakat, M.A. Butterfly cluster like lamellar BiOBr/TiO2 nanocomposite for enhanced sunlight photocatalytic mineralization of aqueous ciprofloxacin. Sci. Total Environ. 2019, 665, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Villaluz, F.D.A.; de Luna, M.D.G.; Colades, J.I.; Garcia-Segura, S.; Lu, M.C. Removal of 4-chlorophenol by visible-light photocatalysis using ammonium iron (II) sulfate-doped nano-titania. Process Saf. Environ. Prot. 2019, 125, 121–128. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; An, T.; Li, G.; Yip, H.-Y.; Wong, P.-K. Comparative study of visible-light-driven photocatalytic mechanisms of dye decolorization and bacterial disinfection by B–Ni-codoped TiO2 microspheres: The role of different reactive species. Appl. Catal. B Environ. 2011, 108–109, 108–116. [Google Scholar] [CrossRef]
- Gao, F.; Jiang, J.; Du, L.; Liu, X.; Ding, Y. Stable and highly efficient Cu/TiO2 nanocomposite photocatalyst prepared through atomic layer deposition. Appl. Catal. A Gen. 2018, 568, 168–175. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Y.; Zhang, M.; Yang, M.; Gong, X.; Yu, F.; Zheng, J. Comparative Study of Graphene Hydrogels and Aerogels Reveals the Important Role of Buried Water in Pollutant Adsorption. Environ. Sci. Technol. 2017, 51, 12283–12292. [Google Scholar] [CrossRef]
- Yadav, N.; Lochab, B. A Comparative Study of Graphene Oxide: Hummers, Intermediate and Improved method. FlatChem 2019. [Google Scholar] [CrossRef]
- Zhou, K.F.; Zhu, Y.H.; Yang, X.L.; Jiang, X.; Li, C.Z. Preparation of graphene-TiO2 composites with enhanced photocatalytic activity. New J. Chem. 2011, 35, 353–359. [Google Scholar] [CrossRef]









| Sample | BET (m2/g) | TPA (mL/g) | dmean (nm) |
|---|---|---|---|
| Pure P25 | 41.628 | 0.2717 | 26.111 |
| 1% rGO–P25 | 52.174 | 0.3421 | 26.224 |
| 5% rGO–P25 | 40.816 | 0.4058 | 39.729 |
| 10% rGO–P25 | 44.581 | 0.3685 | 33.063 |
| Photocatalyst | Pollution | % Degradation | Time (min) | Light Source | Reference |
|---|---|---|---|---|---|
| 1% rGO–P25 | BPA | 100 | 30 | Sunlight | This Work |
| Cu2ZnSnS4 | Phthalic acid | 56 | 240 | Sunlight | [56] |
| NiO | MB | 94 | 120 | Sunlight | [57] |
| Cot–g–Si/Ag@ZnO | RhB | 98 | 40 | the Xenon Lamp | [58] |
| Ni0.6Co0.4O | MB | 95 | 50 | Sunlight | [59] |
| RGO–TiO2 | Cr | 90 | 100 | Simulated Sunlight | [60] |
| pumice-PSCT | MB | 60 | 30 | Simulated Sunlight | [61] |
| MoS2/TiO2 | MB | 90 | 90 | the Xenon Lamp | [62] |
| 15% BiOBr/TiO2 | CIP | 100 | 180 | Sunlight | [63] |
| Fe/N/S–TiO2 | 4-chlorophenol | 100 | 180 | Simulated Sunlight | [64] |
| Solvent | Pure Water:Ethanol = 2:1 | Pure Water | ||||||
|---|---|---|---|---|---|---|---|---|
| Graphene content | 10% | 5% | 3% | 0 | 10% | 5% | 3% | 0 |
| Hydrothermal temperature | 393 K | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Bai, X.; Yang, C.; Xu, L.; Ma, J. Reduced Graphene Oxide–P25 Nanocomposites as Efficient Photocatalysts for Degradation of Bisphenol A in Water. Catalysts 2019, 9, 607. https://doi.org/10.3390/catal9070607
Yu F, Bai X, Yang C, Xu L, Ma J. Reduced Graphene Oxide–P25 Nanocomposites as Efficient Photocatalysts for Degradation of Bisphenol A in Water. Catalysts. 2019; 9(7):607. https://doi.org/10.3390/catal9070607
Chicago/Turabian StyleYu, Fei, Xueting Bai, Changfu Yang, Lijie Xu, and Jie Ma. 2019. "Reduced Graphene Oxide–P25 Nanocomposites as Efficient Photocatalysts for Degradation of Bisphenol A in Water" Catalysts 9, no. 7: 607. https://doi.org/10.3390/catal9070607